当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for the Allosteric Regulation of the SbtA Bicarbonate Transporter by the PII-like Protein, SbtB, from Cyanobium sp. PCC7001.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.biochem.9b00880 Joe A Kaczmarski 1 , Nan-Sook Hong 1 , Bratati Mukherjee 2 , Laura T Wey 2 , Loraine Rourke 2 , Britta Förster 2 , Thomas S Peat 3 , G Dean Price 2 , Colin J Jackson 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.biochem.9b00880 Joe A Kaczmarski 1 , Nan-Sook Hong 1 , Bratati Mukherjee 2 , Laura T Wey 2 , Loraine Rourke 2 , Britta Förster 2 , Thomas S Peat 3 , G Dean Price 2 , Colin J Jackson 1
Affiliation
|
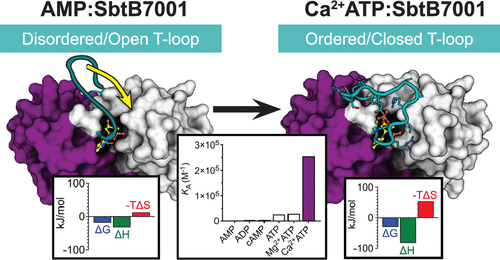
Cyanobacteria have evolved a suite of enzymes and inorganic carbon (Ci) transporters that improve photosynthetic performance by increasing the localized concentration of CO2 around the primary CO2-fixating enzyme, Rubisco. This CO2-concentrating mechanism (CCM) is highly regulated, responds to illumination/darkness cycles, and allows cyanobacteria to thrive under limiting Ci conditions. While the transcriptional control of CCM activity is well understood, less is known about how regulatory proteins might allosterically regulate Ci transporters in response to changing conditions. Cyanobacterial sodium-dependent bicarbonate transporters (SbtAs) are inhibited by PII-like regulatory proteins (SbtBs), with the inhibitory effect being modulated by adenylnucleotides. Here, we used isothermal titration calorimetry to show that SbtB from Cyanobium sp. PCC7001 (SbtB7001) binds AMP, ADP, cAMP, and ATP with micromolar-range affinities. X-ray crystal structures of apo and nucleotide-bound SbtB7001 revealed that while AMP, ADP, and cAMP have little effect on the SbtB7001 structure, binding of ATP stabilizes the otherwise flexible T-loop, and that the flexible C-terminal C-loop adopts several distinct conformations. We also show that ATP binding affinity is increased 10-fold in the presence of Ca2+, and we present an X-ray crystal structure of Ca2+ATP:SbtB7001 that shows how this metal ion facilitates additional stabilizing interactions with the apex of the T-loop. We propose that the Ca2+ATP-induced conformational change observed in SbtB7001 is important for allosteric regulation of SbtA activity by SbtB and is consistent with changing adenylnucleotide levels in illumination/darkness cycles.
中文翻译:

蓝藻属物种的PII样蛋白SbtB对SbtA碳酸氢盐转运蛋白的变构调节的结构基础。PCC7001。
蓝细菌已经进化出一套酶和无机碳(Ci)转运蛋白,它们通过增加主要的CO2固定酶Rubisco周围的局部CO2浓度来改善光合作用。此CO2浓缩机制(CCM)受到高度调节,可响应光照/黑暗周期,并允许蓝细菌在有限的Ci条件下conditions壮成长。尽管对CCM活性的转录控制已广为人知,但对于调节蛋白如何响应条件变化而变构地调节Ci转运蛋白的了解却很少。蓝藻酸钠依赖性碳酸氢盐转运蛋白(SbtAs)被PII样调节蛋白(SbtBs)抑制,抑制作用受腺苷酸核苷酸调节。在这里,我们使用等温滴定热法显示了来自Cyanobium sp。的SbtB。PCC7001(SbtB7001)以微摩尔范围的亲和力结合AMP,ADP,cAMP和ATP。载脂蛋白和与核苷酸结合的SbtB7001的X射线晶体结构表明,虽然AMP,ADP和cAMP对SbtB7001结构影响很小,但ATP的结合稳定了原本灵活的T环,以及灵活的C端C环采用几种不同的构象。我们还显示了在Ca2 +存在下ATP结合亲和力增加了10倍,并且我们展示了Ca2 + ATP:SbtB7001的X射线晶体结构,表明该金属离子如何促进与T-顶点的其他稳定化相互作用环形。我们建议在SbtB7001中观察到的Ca2 + ATP诱导的构象变化对于SbtB的SbtA变构调节SbtA活性很重要,并且与光照/黑暗周期中腺苷酸水平的变化一致。
更新日期:2019-12-04
中文翻译:

蓝藻属物种的PII样蛋白SbtB对SbtA碳酸氢盐转运蛋白的变构调节的结构基础。PCC7001。
蓝细菌已经进化出一套酶和无机碳(Ci)转运蛋白,它们通过增加主要的CO2固定酶Rubisco周围的局部CO2浓度来改善光合作用。此CO2浓缩机制(CCM)受到高度调节,可响应光照/黑暗周期,并允许蓝细菌在有限的Ci条件下conditions壮成长。尽管对CCM活性的转录控制已广为人知,但对于调节蛋白如何响应条件变化而变构地调节Ci转运蛋白的了解却很少。蓝藻酸钠依赖性碳酸氢盐转运蛋白(SbtAs)被PII样调节蛋白(SbtBs)抑制,抑制作用受腺苷酸核苷酸调节。在这里,我们使用等温滴定热法显示了来自Cyanobium sp。的SbtB。PCC7001(SbtB7001)以微摩尔范围的亲和力结合AMP,ADP,cAMP和ATP。载脂蛋白和与核苷酸结合的SbtB7001的X射线晶体结构表明,虽然AMP,ADP和cAMP对SbtB7001结构影响很小,但ATP的结合稳定了原本灵活的T环,以及灵活的C端C环采用几种不同的构象。我们还显示了在Ca2 +存在下ATP结合亲和力增加了10倍,并且我们展示了Ca2 + ATP:SbtB7001的X射线晶体结构,表明该金属离子如何促进与T-顶点的其他稳定化相互作用环形。我们建议在SbtB7001中观察到的Ca2 + ATP诱导的构象变化对于SbtB的SbtA变构调节SbtA活性很重要,并且与光照/黑暗周期中腺苷酸水平的变化一致。









































 京公网安备 11010802027423号
京公网安备 11010802027423号