当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pandonodin: A Proteobacterial Lasso Peptide with an Exceptionally Long C-Terminal Tail.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-12-05 , DOI: 10.1021/acschembio.9b00676 Wai Ling Cheung-Lee 1 , Li Cao 1 , A James Link 1, 2, 3
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-12-05 , DOI: 10.1021/acschembio.9b00676 Wai Ling Cheung-Lee 1 , Li Cao 1 , A James Link 1, 2, 3
Affiliation
|
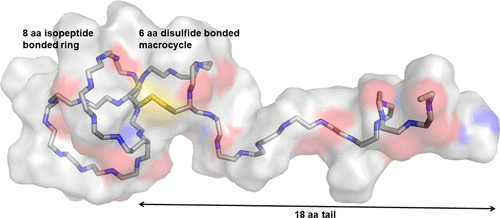
Lasso peptides are a family of ribosomally synthesized and post-translationally modified peptides (RiPPs) defined by their threaded-ring topology. The N-terminus of the peptide forms an isopeptide bond with an aspartate or glutamate side chain to create a 7-9 amino acid (aa) macrocyclic ring through which the rest of the peptide is threaded. The result is a highly constrained three-dimensional structure. Even though they share a threaded-ring feature, characterized lasso peptides vary greatly in sequence and size, ranging from 14 to 26 aa. Using genome mining, we identified a new lasso peptide gene cluster with a predicted lasso peptide that is 33 aa long. Here we report the heterologous expression of this new peptide, pandonodin, its NMR structure, and its unusual biophysical properties. Pandonodin has a long, proteolytically resistant 18-residue tail of low sequence complexity, which limits its water solubility. Within this tail is a 6 aa disulfide-bonded macrocycle that serves as a steric lock to maintain the lasso structure. This disulfide bond is unusually stable, requiring both heat and high concentrations of reductants for cleavage. Finally, we also show that segments of the C-terminal tail of pandonodin can be replaced with arbitrary sequences, allowing for the construction of pandonodin-protein fusions.
中文翻译:

Pandonodin:具有超长C末端尾巴的Proteobacterial Lasso肽。
套索肽是由核糖体合成的和翻译后修饰的肽(RiPP)的家族,它们由其螺纹环拓扑结构定义。肽的N末端与天冬氨酸或谷氨酸的侧链形成异肽键,以形成7-9个氨基酸(aa)的大环,其余的肽则通过该环。结果是高度受约束的三维结构。即使它们具有螺纹环特征,特征化的套索肽的序列和大小也有很大差异,范围从14至26aa。使用基因组挖掘,我们确定了一个新的套索肽基因簇,其预测的套索肽长度为33 aa。在这里,我们报告了这种新肽潘多诺定的异源表达,其NMR结构及其不寻常的生物物理特性。Pandonodin长,具有低序列复杂性的蛋白水解抗性18残基尾部,限制了其水溶性。在该尾部内是一个6aa二硫键结合的大环,可作为维持套索结构的空间锁定。该二硫键异常稳定,需要热量和高浓度的还原剂才能裂解。最后,我们还表明,可以用任意序列替换pandonodin C末端尾巴的片段,从而可以构建pandonodin-蛋白质融合蛋白。
更新日期:2019-12-06
中文翻译:

Pandonodin:具有超长C末端尾巴的Proteobacterial Lasso肽。
套索肽是由核糖体合成的和翻译后修饰的肽(RiPP)的家族,它们由其螺纹环拓扑结构定义。肽的N末端与天冬氨酸或谷氨酸的侧链形成异肽键,以形成7-9个氨基酸(aa)的大环,其余的肽则通过该环。结果是高度受约束的三维结构。即使它们具有螺纹环特征,特征化的套索肽的序列和大小也有很大差异,范围从14至26aa。使用基因组挖掘,我们确定了一个新的套索肽基因簇,其预测的套索肽长度为33 aa。在这里,我们报告了这种新肽潘多诺定的异源表达,其NMR结构及其不寻常的生物物理特性。Pandonodin长,具有低序列复杂性的蛋白水解抗性18残基尾部,限制了其水溶性。在该尾部内是一个6aa二硫键结合的大环,可作为维持套索结构的空间锁定。该二硫键异常稳定,需要热量和高浓度的还原剂才能裂解。最后,我们还表明,可以用任意序列替换pandonodin C末端尾巴的片段,从而可以构建pandonodin-蛋白质融合蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号