当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vitro Human Liver Model of Nonalcoholic Steatohepatitis by Coculturing Hepatocytes, Endothelial Cells, and Kupffer Cells.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-11-20 , DOI: 10.1002/adhm.201901379 Ceri-Anne E Suurmond 1, 2, 3 , Soufian Lasli 1, 2, 4 , Floor W van den Dolder 1, 2, 5, 6 , Aly Ung 1, 2 , Han-Jun Kim 1, 2 , Praveen Bandaru 1, 2 , KangJu Lee 1, 2 , Hyun-Jong Cho 1, 2, 7 , Samad Ahadian 1, 2 , Nureddin Ashammakhi 2, 8 , Mehmet R Dokmeci 2, 8 , Junmin Lee 1, 2 , Ali Khademhosseini 1, 2, 8, 9
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-11-20 , DOI: 10.1002/adhm.201901379 Ceri-Anne E Suurmond 1, 2, 3 , Soufian Lasli 1, 2, 4 , Floor W van den Dolder 1, 2, 5, 6 , Aly Ung 1, 2 , Han-Jun Kim 1, 2 , Praveen Bandaru 1, 2 , KangJu Lee 1, 2 , Hyun-Jong Cho 1, 2, 7 , Samad Ahadian 1, 2 , Nureddin Ashammakhi 2, 8 , Mehmet R Dokmeci 2, 8 , Junmin Lee 1, 2 , Ali Khademhosseini 1, 2, 8, 9
Affiliation
|
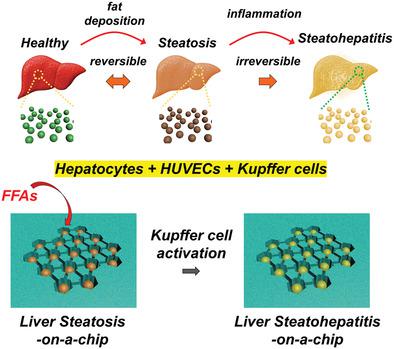
The liver has a complex and unique microenvironment with multiple cell-cell interactions and internal vascular networks. Although nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease with multiple phases, no proper model could fully recapitulate the in vivo microenvironment to understand NAFLD progression. Here, an in vitro human liver model of NAFLD by coculturing human hepatocytes, umbilical vein endothelial cells (HUVECs), and Kupffer cells (KCs) into spheroids is presented. Analysis of indirect cross-talk using conditioned media between steatotic spheroids-composed of hepatocellular carcinoma-derived cells (HepG2) and HUVECs-and mouse KCs reveals that the latter can be activated showing increased cell area, elevated production of reactive oxygen species (ROS), and proinflammatory cytokines. Spheroids incorporating human KCs (HKCs) can also be induced into steatotic stage by supplementing fat. Steatotic spheroids with/without HKCs show different levels of steatotic stages through lipid accumulation and ROS production. Steatotic spheroids made from an immortalized hepatic progenitor cell line (HepaRG) compared to those made from HepG2 cells display similar trends of functionality, but elevated levels of proinflammatory cytokines, and improved reversibility of steatosis. The in vitro human liver system proposed makes strides in developing a model to mimic and monitor the progression of NAFLD.
中文翻译:

通过共培养肝细胞,内皮细胞和库普弗细胞的非酒精性脂肪性肝炎的体外人肝模型。
肝脏具有复杂而独特的微环境,具有多种细胞间相互作用和内部血管网络。尽管非酒精性脂肪肝疾病(NAFLD)是具有多个阶段的最常见的慢性肝病,但没有合适的模型可以完全概括体内微环境以了解NAFLD的进展。在这里,提出了通过将人肝细胞,脐静脉内皮细胞(HUVEC)和库普弗细胞(KC)共培养成球体的NAFLD体外人肝模型。使用条件细胞对肝细胞癌衍生细胞(HepG2)和HUVECs组成的类脂球体和小鼠KCs之间的间接串扰进行分析,结果表明后者可以被激活,显示出细胞面积增加,活性氧(ROS)产生增加和促炎细胞因子。掺入人KCs(HKCs)的球状体也可以通过补充脂肪而诱导进入脂肪变性阶段。带有/不带有HKC的类脂球体通过脂质积累和ROS产生显示出不同水平的类脂阶段。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。
更新日期:2019-12-19
中文翻译:

通过共培养肝细胞,内皮细胞和库普弗细胞的非酒精性脂肪性肝炎的体外人肝模型。
肝脏具有复杂而独特的微环境,具有多种细胞间相互作用和内部血管网络。尽管非酒精性脂肪肝疾病(NAFLD)是具有多个阶段的最常见的慢性肝病,但没有合适的模型可以完全概括体内微环境以了解NAFLD的进展。在这里,提出了通过将人肝细胞,脐静脉内皮细胞(HUVEC)和库普弗细胞(KC)共培养成球体的NAFLD体外人肝模型。使用条件细胞对肝细胞癌衍生细胞(HepG2)和HUVECs组成的类脂球体和小鼠KCs之间的间接串扰进行分析,结果表明后者可以被激活,显示出细胞面积增加,活性氧(ROS)产生增加和促炎细胞因子。掺入人KCs(HKCs)的球状体也可以通过补充脂肪而诱导进入脂肪变性阶段。带有/不带有HKC的类脂球体通过脂质积累和ROS产生显示出不同水平的类脂阶段。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。与由HepG2细胞制成的永生化肝祖细胞系(HepaRG)所制成的类脂球体显示出相似的功能趋势,但促炎性细胞因子水平升高,并改善了脂肪变性的可逆性。拟议的体外人类肝脏系统在开发模拟和监测NAFLD进程的模型方面取得了长足的进步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号