当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal-Organic Framework Nanoparticles to Boost Cancer Immunotherapy.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-11-20 , DOI: 10.1002/adhm.201900996 Zhixiong Cai 1 , Fuli Xin 2, 3 , Zuwu Wei 2, 3 , Ming Wu 2, 3 , Xinyi Lin 1 , Xiaofan Du 1 , Geng Chen 1 , Da Zhang 2, 3 , Zhenxi Zhang 1 , Xiaolong Liu 2, 3 , Cuiping Yao 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2019-11-20 , DOI: 10.1002/adhm.201900996 Zhixiong Cai 1 , Fuli Xin 2, 3 , Zuwu Wei 2, 3 , Ming Wu 2, 3 , Xinyi Lin 1 , Xiaofan Du 1 , Geng Chen 1 , Da Zhang 2, 3 , Zhenxi Zhang 1 , Xiaolong Liu 2, 3 , Cuiping Yao 1
Affiliation
|
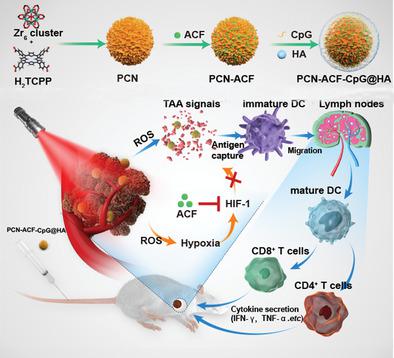
Photodynamic therapy (PDT) usually aggravates tumor hypoxia, which promotes the survival and metastasis of residue cancer cells; furthermore, although PDT-induced immunogenic death of cancer cells can induce host antitumor responses, such responses are generally weak and not enough to eliminate the residue cancer cells. Here, metal-organic framework (MOF)-based nanoparticles to combine PDT, antihypoxic signaling, and CpG adjuvant as an in situ tumor vaccine to boost host anticancer responses after PDT are designed. The MOF-based nanoparticles are self-assembled from H2 TCPP and zirconium ions with hypoxia inducible factor (HIF) signaling inhibitor (ACF) and immunologic adjuvant (CpG) loading, and hyaluronic acid (HA) coating on the surface. The final nanoparticles (PCN-ACF-CpG@HA) can specifically target cancer cells overexpressing CD44 receptor though HA; the aggravated hypoxic survival signaling after PDT can be blocked by ACF to inhibit the HIF-1α induced survival and metastasis. With the help of CpG adjuvant, the tumor associated antigens generated from PDT-based cancer cell destruction can initiate strong antitumor immune responses to eliminate residue cancer cells. Taken together, a novel in situ immunostimulatory strategy is designed to synergistically enhance therapeutic effects of PDT by activating host antitumor immune-responses both in vitro and in vivo, which may have great potential for clinical translation in future.
中文翻译:

光动力疗法结合抗氧信号和CpG佐剂作为基于金属-有机骨架纳米粒子的原位肿瘤疫苗,以促进癌症的免疫治疗。
光动力疗法(PDT)通常会加重肿瘤缺氧,从而促进残留癌细胞的存活和转移。此外,尽管PDT诱导的癌细胞的免疫原性死亡可以诱导宿主抗肿瘤反应,但是这种反应通常较弱并且不足以消除残留的癌细胞。在这里,设计了基于金属有机框架(MOF)的纳米颗粒,将PDT,抗氧信号和CpG佐剂结合起来作为原位肿瘤疫苗,以增强PDT后的宿主抗癌反应。基于MOF的纳米颗粒由H2 TCPP和锆离子自组装而成,表面具有缺氧诱导因子(HIF)信号抑制剂(ACF)和免疫佐剂(CpG),并有透明质酸(HA)涂层。最终的纳米粒子(PCN-ACF-CpG @ HA)可以通过HA特异性靶向过度表达CD44受体的癌细胞;ACF可以阻断PDT后加重的低氧生存信号,从而抑制HIF-1α诱导的生存和转移。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在未来的临床翻译中可能具有很大的潜力。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在将来可能具有很大的临床翻译潜力。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在未来的临床翻译中可能具有很大的潜力。
更新日期:2020-01-08
中文翻译:

光动力疗法结合抗氧信号和CpG佐剂作为基于金属-有机骨架纳米粒子的原位肿瘤疫苗,以促进癌症的免疫治疗。
光动力疗法(PDT)通常会加重肿瘤缺氧,从而促进残留癌细胞的存活和转移。此外,尽管PDT诱导的癌细胞的免疫原性死亡可以诱导宿主抗肿瘤反应,但是这种反应通常较弱并且不足以消除残留的癌细胞。在这里,设计了基于金属有机框架(MOF)的纳米颗粒,将PDT,抗氧信号和CpG佐剂结合起来作为原位肿瘤疫苗,以增强PDT后的宿主抗癌反应。基于MOF的纳米颗粒由H2 TCPP和锆离子自组装而成,表面具有缺氧诱导因子(HIF)信号抑制剂(ACF)和免疫佐剂(CpG),并有透明质酸(HA)涂层。最终的纳米粒子(PCN-ACF-CpG @ HA)可以通过HA特异性靶向过度表达CD44受体的癌细胞;ACF可以阻断PDT后加重的低氧生存信号,从而抑制HIF-1α诱导的生存和转移。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在未来的临床翻译中可能具有很大的潜力。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在将来可能具有很大的临床翻译潜力。借助CpG佐剂,基于PDT的癌细胞破坏产生的与肿瘤相关的抗原可以启动强大的抗肿瘤免疫反应,从而消除残留的癌细胞。综上所述,设计了一种新颖的原位免疫刺激策略,以通过在体外和体内激活宿主抗肿瘤免疫应答来协同增强PDT的治疗效果,这在未来的临床翻译中可能具有很大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号