当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rap1-mediated nucleosome displacement can regulate gene expression in senescent cells without impacting the pace of senescence.
Aging Cell ( IF 7.8 ) Pub Date : 2019-11-19 , DOI: 10.1111/acel.13061 Shufei Song 1, 2, 3 , Javier V Perez 3 , William Svitko 3 , M Daniel Ricketts 1, 4 , Elliot Dean 5 , David Schultz 5 , Ronen Marmorstein 1, 4 , F Brad Johnson 1, 3, 6
Aging Cell ( IF 7.8 ) Pub Date : 2019-11-19 , DOI: 10.1111/acel.13061 Shufei Song 1, 2, 3 , Javier V Perez 3 , William Svitko 3 , M Daniel Ricketts 1, 4 , Elliot Dean 5 , David Schultz 5 , Ronen Marmorstein 1, 4 , F Brad Johnson 1, 3, 6
Affiliation
|
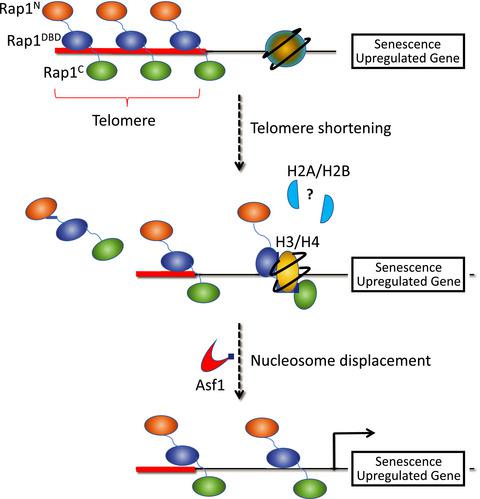
Cell senescence is accompanied, and in part mediated, by changes in chromatin, including histone losses, but underlying mechanisms are not well understood. We reported previously that during yeast cell senescence driven by telomere shortening, the telomeric protein Rap1 plays a major role in reprogramming gene expression by relocalizing hundreds of new target genes (called NRTS, for new Rap1 targets at senescence) to the promoters. This leads to two types of histone loss: Rap1 lowers histone level globally by repressing histone gene expression, and it also causes local nucleosome displacement at the promoters of upregulated NRTS. Here, we present evidence of direct binding between Rap1 and histone H3/H4 heterotetramers, and map amino acids involved in the interaction within the Rap1 SANT domain to amino acids 392–394 (SHY). Introduction of a point mutation within the native RAP1 locus that converts these residues to alanines (RAP1SHY), and thus disrupts Rap1‐H3/H4 interaction, does not interfere with Rap1 relocalization to NRTS at senescence, but prevents full nucleosome displacement and gene upregulation, indicating direct Rap1‐H3/H4 contacts are involved in nucleosome displacement. Consistent with this, the histone H3/H4 chaperone Asf1 is similarly unnecessary for Rap1 localization to NRTS but is required for full Rap1‐mediated nucleosome displacement and gene activation. Remarkably, RAP1SHY does not affect the pace of senescence‐related cell cycle arrest, indicating that some changes in gene expression at senescence are not coupled to this arrest.
中文翻译:

Rap1介导的核小体置换可以调节衰老细胞中的基因表达,而不会影响衰老的速度。
细胞衰老伴随着染色质的变化,包括组蛋白丢失,但部分介导了染色质的变化,但其潜在机制尚不十分清楚。我们以前报道,端粒缩短驱动的酵母细胞衰老过程中,端粒蛋白的Rap1起着通过relocalizing数百个新的靶基因(称为NRTS,用于重编程的基因表达中起主要作用ň EW [R AP1牛逼的具体目标小号(启动子)。这导致两种类型的组蛋白丢失:Rap1通过抑制组蛋白基因表达来整体降低组蛋白水平,并且还导致上调NRTS启动子处的局部核小体置换。在这里,我们提供Rap1和组蛋白H3 / H4异四聚体之间直接结合的证据,并将Rap1 SANT域内相互作用中涉及的氨基酸定位为氨基酸392-394(SHY)。在天然RAP1基因座中引入点突变,将这些残基转化为丙氨酸(RAP1 SHY),从而破坏Rap1-H3 / H4的相互作用,在衰老时不干扰Rap1重新定位至NRTS,但阻止了核小体的完全置换和基因上调,表明Rap1-H3 / H4直接接触参与了核小体的置换。与此相一致,组蛋白H3 / H4分子伴侣Asf1对于Rap1定位到NRTS同样是不必要的,但对于Rap1介导的核小体的完全置换和基因激活是必需的。值得注意的是,RAP1 SHY不会影响衰老相关的细胞周期停滞的速度,这表明衰老时基因表达的某些变化与该停滞不相关。
更新日期:2019-11-19
中文翻译:

Rap1介导的核小体置换可以调节衰老细胞中的基因表达,而不会影响衰老的速度。
细胞衰老伴随着染色质的变化,包括组蛋白丢失,但部分介导了染色质的变化,但其潜在机制尚不十分清楚。我们以前报道,端粒缩短驱动的酵母细胞衰老过程中,端粒蛋白的Rap1起着通过relocalizing数百个新的靶基因(称为NRTS,用于重编程的基因表达中起主要作用ň EW [R AP1牛逼的具体目标小号(启动子)。这导致两种类型的组蛋白丢失:Rap1通过抑制组蛋白基因表达来整体降低组蛋白水平,并且还导致上调NRTS启动子处的局部核小体置换。在这里,我们提供Rap1和组蛋白H3 / H4异四聚体之间直接结合的证据,并将Rap1 SANT域内相互作用中涉及的氨基酸定位为氨基酸392-394(SHY)。在天然RAP1基因座中引入点突变,将这些残基转化为丙氨酸(RAP1 SHY),从而破坏Rap1-H3 / H4的相互作用,在衰老时不干扰Rap1重新定位至NRTS,但阻止了核小体的完全置换和基因上调,表明Rap1-H3 / H4直接接触参与了核小体的置换。与此相一致,组蛋白H3 / H4分子伴侣Asf1对于Rap1定位到NRTS同样是不必要的,但对于Rap1介导的核小体的完全置换和基因激活是必需的。值得注意的是,RAP1 SHY不会影响衰老相关的细胞周期停滞的速度,这表明衰老时基因表达的某些变化与该停滞不相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号