当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterising the brain metalloproteome in Down syndrome patients with concomitant Alzheimer's pathology.
Metallomics ( IF 2.9 ) Pub Date : 2019-11-18 , DOI: 10.1039/c9mt00196d Nakisa Malakooti 1 , Blaine Roberts , Melanie A Pritchard , Irene Volitakis , Ron C Kim , Ira T Lott , Catriona A McLean , David I Finkelstein , Paul A Adlard
Metallomics ( IF 2.9 ) Pub Date : 2019-11-18 , DOI: 10.1039/c9mt00196d Nakisa Malakooti 1 , Blaine Roberts , Melanie A Pritchard , Irene Volitakis , Ron C Kim , Ira T Lott , Catriona A McLean , David I Finkelstein , Paul A Adlard
Affiliation
|
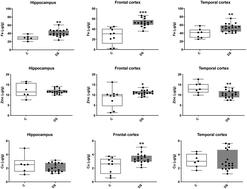
Down syndrome (DS) is a common intellectual disability, with an incidence of 1 in 700 and is caused by trisomy 21. People with DS develop Alzheimer's disease (AD)-like neuropathology by the age of 40. As metal ion dyshomeostasis (particularly zinc, iron and copper) is one of the characteristics of AD and is believed to be involved in the pathogenesis of disease, we reasoned that it may also be altered in DS. Thus, we used inductively coupled plasma mass spectrometry to examine metal levels in post-mortem brain tissue from DS individuals with concomitant AD pathology. Size exclusion-ICPMS was also utilised to characterise the metalloproteome in these cases. We report here for the first time that iron levels were higher in a number of regions in the DS brain, including the hippocampus (40%), frontal cortex (100%) and temporal cortex (34%), compared to controls. Zinc and copper were also elevated (both 29%) in the DS frontal cortex, but zinc was decreased (23%) in the DS temporal cortex. Other elements were also examined, a number of which also showed disease-specific changes. The metalloproteomic profile in the DS brain was also different to that in the controls. These data suggest that metals and metal:protein interactions are dysregulated in the DS brain which, given the known role of metals in neurodegeneration and AD, is likely to contribute to the pathogenesis of disease. Interrogation of the underlying cellular mechanisms and consequences of this failure in metal ion homeostasis, and the specific contributions of the individual DS and AD phenotypes to these changes, should be explored.
中文翻译:

唐氏综合症患者伴有阿尔茨海默氏病的脑金属蛋白质组学特征。
唐氏综合症(DS)是一种常见的智力障碍,发病率700分之一,由21三体性疾病引起。DS患者到40岁时会发展为类似阿尔茨海默氏病(AD)的神经病理。 (铁和铜)是AD的特征之一,并且被认为与疾病的发病机制有关,我们认为在DS中也可能发生改变。因此,我们使用电感耦合等离子体质谱法检查了DS伴有AD病理的死后脑组织中的金属水平。在这些情况下,尺寸排阻-ICPMS还用于表征金属蛋白质组。我们首次在此报告DS大脑中多个区域的铁水平较高,包括海马(40%),额叶皮层(100%)和颞叶皮层(34%),与控件相比。DS额叶皮层中的锌和铜也升高(均为29%),但DS颞叶皮层中的锌减少(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。但锌在颞叶皮质中减少了(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。但锌在颞叶皮质中减少了(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。
更新日期:2019-11-18
中文翻译:

唐氏综合症患者伴有阿尔茨海默氏病的脑金属蛋白质组学特征。
唐氏综合症(DS)是一种常见的智力障碍,发病率700分之一,由21三体性疾病引起。DS患者到40岁时会发展为类似阿尔茨海默氏病(AD)的神经病理。 (铁和铜)是AD的特征之一,并且被认为与疾病的发病机制有关,我们认为在DS中也可能发生改变。因此,我们使用电感耦合等离子体质谱法检查了DS伴有AD病理的死后脑组织中的金属水平。在这些情况下,尺寸排阻-ICPMS还用于表征金属蛋白质组。我们首次在此报告DS大脑中多个区域的铁水平较高,包括海马(40%),额叶皮层(100%)和颞叶皮层(34%),与控件相比。DS额叶皮层中的锌和铜也升高(均为29%),但DS颞叶皮层中的锌减少(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。但锌在颞叶皮质中减少了(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。但锌在颞叶皮质中减少了(23%)。还检查了其他元素,其中许多元素也显示出特定于疾病的变化。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。DS大脑中的金属古生物学特征也与对照者不同。这些数据表明,DS脑中金属和金属:蛋白质的相互作用失调,鉴于金属在神经退行性疾病和AD中的已知作用,DS脑中可能与疾病的发病机理有关。应该探讨对潜在细胞机制的询问以及这种失败在金属离子稳态中的后果,以及各个DS和AD表型对这些变化的特定贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号