Cell Discovery ( IF 13.0 ) Pub Date : 2019-11-19 , DOI: 10.1038/s41421-019-0131-9 Chun-Seok Cho , Allison H. Kowalsky , Sim Namkoong , Sung-Rye Park , Shuangcheng Wu , Boyoung Kim , Amanda James , Bondong Gu , Ian A. Semple , Mohamed A. Tohamy , Sumeet Solanki , Uhn-Soo Cho , Joel K. Greenson , Yatrik M. Shah , Myungjin Kim , Jun Hee Lee
|
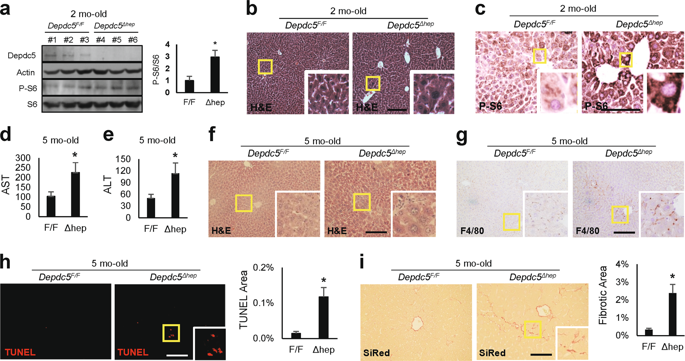
mTORC1 is a protein kinase important for metabolism and is regulated by growth factor and nutrient signaling pathways, mediated by the Rheb and Rag GTPases, respectively. Here we provide the first animal model in which both pathways were upregulated through concurrent mutations in their GTPase-activating proteins, Tsc1 and Depdc5. Unlike former models that induced limited mTORC1 upregulation, hepatic deletion of both Tsc1 and Depdc5 (DKO) produced strong, synergistic activation of the mTORC1 pathway and provoked pronounced and widespread hepatocyte damage, leading to externally visible liver failure phenotypes, such as jaundice and systemic growth defects. The transcriptome profile of DKO was different from single knockout mutants but similar to those of diseased human livers with severe hepatitis and mouse livers challenged with oxidative stress-inducing chemicals. In addition, DKO liver cells exhibited prominent molecular pathologies associated with excessive endoplasmic reticulum (ER) stress, oxidative stress, DNA damage and inflammation. Although DKO liver pathologies were ameliorated by mTORC1 inhibition, ER stress suppression unexpectedly aggravated them, suggesting that ER stress signaling is not the major conduit of how hyperactive mTORC1 produces liver damage. Interestingly, superoxide scavengers N-acetylcysteine (NAC) and Tempol, chemicals that reduce oxidative stress, were able to recover liver phenotypes, indicating that mTORC1 hyperactivation induced liver damage mainly through oxidative stress pathways. Our study provides a new model of unregulated mTORC1 activation through concomitant upregulation of growth factor and nutrient signaling axes and shows that mTORC1 hyperactivation alone can provoke oxidative tissue injury.
中文翻译:

mTORC1的生长因子和营养臂的同时激活诱导氧化性肝损伤
mTORC1是一种对新陈代谢很重要的蛋白激酶,并受分别由Rheb和Rag GTPases介导的生长因子和营养信号通路的调节。在这里,我们提供了第一个动物模型,其中两种途径均通过其GTPase激活蛋白Tsc1和Depdc5的并发突变而被上调。与以前的模型诱导mTORC1上调受限不同,Tsc1和Depdc5均被肝删除(DKO)对mTORC1途径产生了强大的协同激活作用,并引发了明显且广泛的肝细胞损伤,导致了外部可见的肝衰竭表型,如黄疸和全身性生长缺陷。DKO的转录组谱不同于单个敲除突变体,但类似于患有严重肝炎的患病人类肝脏和用氧化应激诱导化学物质攻击的小鼠肝脏。此外,DKO肝细胞表现出与过度内质网(ER)应激,氧化应激,DNA损伤和炎症相关的突出分子病理学。尽管通过mTORC1抑制可改善DKO肝脏病理,但ER应激抑制出乎意料地加剧了它们,这表明ER应激信号并不是mTORC1过度活跃如何引起肝损伤的主要途径。有趣的是,减少氧化应激的化学物质超氧化物清除剂N-乙酰半胱氨酸(NAC)和Tempol能够恢复肝表型,表明mTORC1过度活化主要通过氧化应激途径引起肝损伤。我们的研究通过生长因子和营养素信号轴的上调同时提供了不受调节的mTORC1激活的新模型,并表明仅mTORC1过度激活会引起氧化性组织损伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号