当前位置:
X-MOL 学术
›
Hypertens. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brain perivascular macrophages: connecting inflammation to autonomic activity in hypertension
Hypertension Research ( IF 4.3 ) Pub Date : 2019-11-19 , DOI: 10.1038/s41440-019-0359-7 Sébastien Foulquier 1, 2, 3
Hypertension Research ( IF 4.3 ) Pub Date : 2019-11-19 , DOI: 10.1038/s41440-019-0359-7 Sébastien Foulquier 1, 2, 3
Affiliation
|
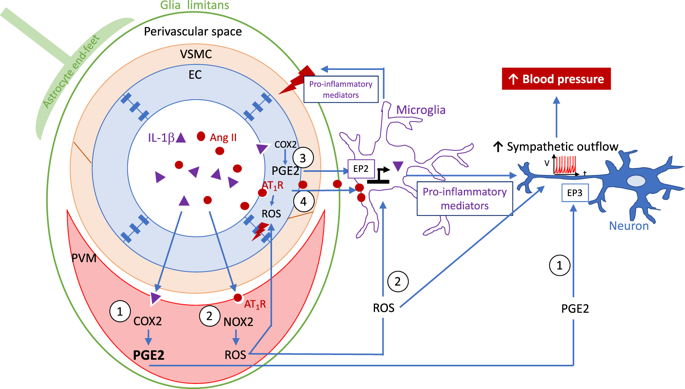
The contribution of the immune system to the pathogenesis of essential hypertension has been documented in many experimental and clinical studies as extensively reviewed elsewhere [1]. In particular, macrophage infiltration into the vascular walls has been shown to be involved in the development of hypertension via the promotion of vascular inflammation and endothelial dysfunction [2, 3]. In a study by Iyonaga et al. [4], the authors described the role of brain perivascular macrophages (PVMs) in the development of hypertension via enhanced sympathetic activation, thereby linking the immune and autonomic systems. Brain PVMs, located in the perivascular space surrounding large cerebral arterioles [5], differ from vessel-associated microglia, which are juxtaposed to all cerebral vessels but located beyond the glia limitans [6]. Brain PVMs have been scrutinized in recent years, as their particular location position them as key players in the initiation of cerebrovascular dysfunction, as shown in hypertensive mice and Alzheimer’s disease mouse models [7, 8]. In their study, the authors demonstrated that IL-1β leads to the overexpression of prostaglandin E2 (PGE2), a known trigger of increased sympathetic outflow in cardiovascular centers, in PVMs [9] (Fig. 1). Furthermore, PVM depletion induced by clodronate liposomes was able to limit the blood pressure increase in stroke-prone spontaneously hypertensive rats. While increased PGE2 expression was observed in PVMs, the potential impact of PGE2 in other brain cells, such as endothelial cells and microglia, cannot be excluded (Fig. 1, routes 1 and 3). The contribution of the central immune system to sympathetic activity is not completely new, as previous studies have shown that the release of proinflammatory cytokines by activated microglia in the paraventricular nucleus (PVN) contributes to neurogenic hypertension [10]. In this study, hypertension was mimicked by the infusion of Angiotensin II (Ang II) for 4 weeks, and the infusion of minocycline (icv, an anti-inflammatory antibiotic) in Ang II-infused rats was able to decrease the number of activated microglia and the expression of proinflammatory cytokines and attenuate the blood pressure elevation [10]. The contribution of PVMs in the later study cannot be excluded, as the detection and quantification methods were not able to distinguish microglia from macrophages. The possible contribution of Ang II was not studied in the study by Iyonaga et al. [4], but Ang II may also be involved in the activation of PVMs and subsequent sympathetic activity, as the Ang II plasma level is known to be elevated in spontaneously hypertensive rats (SHRs) [11, 12]. Ang II-mediated microglial and PVM activation results from the activation of Angiotensin II type 1 receptor (AT1R) [7, 13]. The blockade of AT1R and the stimulation of the counteracting AT2R [14] are known to promote an anti-inflammatory microglial phenotype [15–17]. Angiotensin receptor antagonists may therefore be of potential importance beyond their blood pressure lowering effects and their beneficial impact on the structure and function of cerebral vessels [18, 19]. Although blood-brain barrier (BBB) permeability was not assessed in the present study, previous studies have indicated increased BBB permeability in the PVN, rostral ventrolateral medulla and nucleus tractus solitarius in SHRs and other hypertensive models, which allows the leakage of Ang II in these key sympathoexcitatory brain areas [20] (as illustrated in Fig. 1, routes 2 and 4). While the depletion of PVMs by centrally administered clodronate liposomes has proven to be effective in * Sébastien Foulquier s.foulquier@maastrichtuniversity.nl
中文翻译:

脑血管周围巨噬细胞:将炎症与高血压的自主神经活动联系起来
免疫系统对原发性高血压发病机制的贡献已在许多实验和临床研究中得到证实,并在其他地方进行了广泛审查[1]。特别是,巨噬细胞浸润到血管壁已被证明通过促进血管炎症和内皮功能障碍参与高血压的发展[2, 3]。在 Iyonaga 等人的一项研究中。[4],作者描述了脑血管周围巨噬细胞 (PVM) 通过增强交感神经激活在高血压发展中的作用,从而将免疫系统和自主神经系统联系起来。脑 PVM 位于大脑小动脉周围的血管周围空间 [5],与血管相关小胶质细胞不同,后者与所有脑血管并列,但位于胶质细胞界限之外 [6]。近年来,脑 PVM 受到了仔细研究,因为它们的特殊位置将它们定位为脑血管功能障碍的关键参与者,如高血压小鼠和阿尔茨海默病小鼠模型所示 [7, 8]。在他们的研究中,作者证明 IL-1β 导致 PVM 中前列腺素 E2 (PGE2) 的过度表达,这是已知的心血管中心交感神经外流增加的触发因素 [9](图 1)。此外,氯膦酸盐脂质体诱导的 PVM 耗竭能够限制易中风的自发性高血压大鼠的血压升高。虽然在 PVM 中观察到 PGE2 表达增加,但不能排除 PGE2 在其他脑细胞(如内皮细胞和小胶质细胞)中的潜在影响(图 1,路线 1 和 3)。中枢免疫系统对交感神经活动的贡献并不是全新的,因为先前的研究表明,室旁核 (PVN) 中活化的小胶质细胞释放促炎细胞因子会导致神经源性高血压 [10]。在这项研究中,通过输注血管紧张素 II (Ang II) 4 周来模拟高血压,并且在输注 Ang II 的大鼠中输注米诺环素(icv,一种抗炎抗生素)能够减少活化小胶质细胞的数量和促炎细胞因子的表达并减轻血压升高[10]。不能排除 PVM 在后期研究中的贡献,因为检测和量化方法无法区分小胶质细胞和巨噬细胞。Iyonaga 等人的研究没有研究 Ang II 的可能贡献。[4],但 Ang II 也可能参与 PVM 的激活和随后的交感神经活动,因为已知自发性高血压大鼠 (SHR) 的 Ang II 血浆水平升高 [11, 12]。Ang II 介导的小胶质细胞和 PVM 激活是由血管紧张素 II 1 型受体 (AT1R) 的激活引起的 [7, 13]。已知 AT1R 的阻断和对抗性 AT2R 的刺激 [14] 可促进抗炎小胶质细胞表型 [15-17]。因此,血管紧张素受体拮抗剂的潜在重要性可能超出其降血压作用及其对脑血管结构和功能的有益影响[18, 19]。尽管本研究未评估血脑屏障 (BBB) 通透性,但 先前的研究表明,在 SHR 和其他高血压模型中,PVN、延髓腹外侧和孤束核的 BBB 通透性增加,这使得 Ang II 在这些关键的交感神经兴奋性脑区渗漏 [20](如图 1 所示,路线2 和 4)。虽然通过集中给药的氯膦酸盐脂质体消耗 PVMs 已被证明是有效的 * Sébastien Foulquier s.foulquier@maastrichtuniversity.nl
更新日期:2019-11-19
中文翻译:

脑血管周围巨噬细胞:将炎症与高血压的自主神经活动联系起来
免疫系统对原发性高血压发病机制的贡献已在许多实验和临床研究中得到证实,并在其他地方进行了广泛审查[1]。特别是,巨噬细胞浸润到血管壁已被证明通过促进血管炎症和内皮功能障碍参与高血压的发展[2, 3]。在 Iyonaga 等人的一项研究中。[4],作者描述了脑血管周围巨噬细胞 (PVM) 通过增强交感神经激活在高血压发展中的作用,从而将免疫系统和自主神经系统联系起来。脑 PVM 位于大脑小动脉周围的血管周围空间 [5],与血管相关小胶质细胞不同,后者与所有脑血管并列,但位于胶质细胞界限之外 [6]。近年来,脑 PVM 受到了仔细研究,因为它们的特殊位置将它们定位为脑血管功能障碍的关键参与者,如高血压小鼠和阿尔茨海默病小鼠模型所示 [7, 8]。在他们的研究中,作者证明 IL-1β 导致 PVM 中前列腺素 E2 (PGE2) 的过度表达,这是已知的心血管中心交感神经外流增加的触发因素 [9](图 1)。此外,氯膦酸盐脂质体诱导的 PVM 耗竭能够限制易中风的自发性高血压大鼠的血压升高。虽然在 PVM 中观察到 PGE2 表达增加,但不能排除 PGE2 在其他脑细胞(如内皮细胞和小胶质细胞)中的潜在影响(图 1,路线 1 和 3)。中枢免疫系统对交感神经活动的贡献并不是全新的,因为先前的研究表明,室旁核 (PVN) 中活化的小胶质细胞释放促炎细胞因子会导致神经源性高血压 [10]。在这项研究中,通过输注血管紧张素 II (Ang II) 4 周来模拟高血压,并且在输注 Ang II 的大鼠中输注米诺环素(icv,一种抗炎抗生素)能够减少活化小胶质细胞的数量和促炎细胞因子的表达并减轻血压升高[10]。不能排除 PVM 在后期研究中的贡献,因为检测和量化方法无法区分小胶质细胞和巨噬细胞。Iyonaga 等人的研究没有研究 Ang II 的可能贡献。[4],但 Ang II 也可能参与 PVM 的激活和随后的交感神经活动,因为已知自发性高血压大鼠 (SHR) 的 Ang II 血浆水平升高 [11, 12]。Ang II 介导的小胶质细胞和 PVM 激活是由血管紧张素 II 1 型受体 (AT1R) 的激活引起的 [7, 13]。已知 AT1R 的阻断和对抗性 AT2R 的刺激 [14] 可促进抗炎小胶质细胞表型 [15-17]。因此,血管紧张素受体拮抗剂的潜在重要性可能超出其降血压作用及其对脑血管结构和功能的有益影响[18, 19]。尽管本研究未评估血脑屏障 (BBB) 通透性,但 先前的研究表明,在 SHR 和其他高血压模型中,PVN、延髓腹外侧和孤束核的 BBB 通透性增加,这使得 Ang II 在这些关键的交感神经兴奋性脑区渗漏 [20](如图 1 所示,路线2 和 4)。虽然通过集中给药的氯膦酸盐脂质体消耗 PVMs 已被证明是有效的 * Sébastien Foulquier s.foulquier@maastrichtuniversity.nl











































 京公网安备 11010802027423号
京公网安备 11010802027423号