当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Potent and Selective MTH1 Inhibitors for Oncology: Enabling Rapid Target (In)Validation
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-19 , DOI: 10.1021/acsmedchemlett.9b00420 Julie Farand 1 , Jeffrey E. Kropf 2 , Peter Blomgren 2 , Jianjun Xu 2 , Aaron C. Schmitt 2 , Zachary E. Newby 1 , Ting Wang 1 , Eisuke Murakami 1 , Ona Barauskas 1 , Jawahar Sudhamsu 1 , Joy Y. Feng 1 , Anita Niedziela-Majka 1 , Brian E. Schultz 1 , Karen Schwartz 1 , Serge Viatchenko-Karpinski 1 , Dmytro Kornyeyev 1 , Adam Kashishian 2 , Peidong Fan 1 , Xiaowu Chen 1 , Eric B. Lansdon 1 , Michael O. Ports 2 , Kevin S. Currie 2 , William J. Watkins 1 , Gregory T. Notte 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-19 , DOI: 10.1021/acsmedchemlett.9b00420 Julie Farand 1 , Jeffrey E. Kropf 2 , Peter Blomgren 2 , Jianjun Xu 2 , Aaron C. Schmitt 2 , Zachary E. Newby 1 , Ting Wang 1 , Eisuke Murakami 1 , Ona Barauskas 1 , Jawahar Sudhamsu 1 , Joy Y. Feng 1 , Anita Niedziela-Majka 1 , Brian E. Schultz 1 , Karen Schwartz 1 , Serge Viatchenko-Karpinski 1 , Dmytro Kornyeyev 1 , Adam Kashishian 2 , Peidong Fan 1 , Xiaowu Chen 1 , Eric B. Lansdon 1 , Michael O. Ports 2 , Kevin S. Currie 2 , William J. Watkins 1 , Gregory T. Notte 1
Affiliation
|
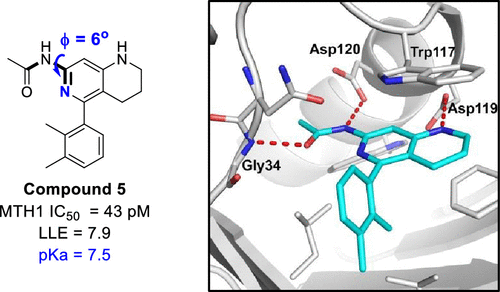
We describe the discovery of three structurally differentiated potent and selective MTH1 inhibitors and their subsequent use to investigate MTH1 as an oncology target, culminating in target (in)validation. Tetrahydronaphthyridine 5 was rapidly identified as a highly potent MTH1 inhibitor (IC50 = 0.043 nM). Cocrystallization of 5 with MTH1 revealed the ligand in a Φ-cis-N-(pyridin-2-yl)acetamide conformation enabling a key intramolecular hydrogen bond and polar interactions with residues Gly34 and Asp120. Modification of literature compound TH287 with O- and N-linked aryl and alkyl aryl substituents led to the discovery of potent pyrimidine-2,4,6-triamine 25 (IC50 = 0.49 nM). Triazolopyridine 32 emerged as a highly selective lead compound with a suitable in vitro profile and desirable pharmacokinetic properties in rat. Elucidation of the DNA damage response, cell viability, and intracellular concentrations of oxo-NTPs (oxidized nucleoside triphosphates) as a function of MTH1 knockdown and/or small molecule inhibition was studied. Based on our findings, we were unable to provide evidence to further pursue MTH1 as an oncology target.
中文翻译:

发现有效的和选择性的肿瘤MTH1抑制剂:启用快速靶标(In)验证
我们描述了三种结构上有区别的有效和选择性MTH1抑制剂的发现,以及它们随后用于研究MTH1作为肿瘤靶标,最终达到靶标(无效)的目的。四氢萘啶5被迅速鉴定为高效MTH1抑制剂(IC 50 = 0.043 nM)。的共结晶5与MTH1揭示在Φ-配体顺式- ñ - (吡啶-2-基)乙酰胺构象使得关键分子内氢键和极性相互作用的残基Gly34和Asp120。用O-和N修饰文献化合物TH287-连接的芳基和烷基芳基取代基导致发现有效的嘧啶-2,4,6-三胺25(IC 50 = 0.49 nM)。三唑并吡啶32以具有高选择性的铅化合物出现,在大鼠中具有合适的体外特性和所需的药代动力学特性。研究了根据MTH1敲低和/或小分子抑制作用来研究DNA损伤反应,细胞活力和氧代-NTPs(氧化的核苷三磷酸)的细胞内浓度。根据我们的发现,我们无法提供证据进一步将MTH1用作肿瘤靶标。
更新日期:2019-11-19
中文翻译:

发现有效的和选择性的肿瘤MTH1抑制剂:启用快速靶标(In)验证
我们描述了三种结构上有区别的有效和选择性MTH1抑制剂的发现,以及它们随后用于研究MTH1作为肿瘤靶标,最终达到靶标(无效)的目的。四氢萘啶5被迅速鉴定为高效MTH1抑制剂(IC 50 = 0.043 nM)。的共结晶5与MTH1揭示在Φ-配体顺式- ñ - (吡啶-2-基)乙酰胺构象使得关键分子内氢键和极性相互作用的残基Gly34和Asp120。用O-和N修饰文献化合物TH287-连接的芳基和烷基芳基取代基导致发现有效的嘧啶-2,4,6-三胺25(IC 50 = 0.49 nM)。三唑并吡啶32以具有高选择性的铅化合物出现,在大鼠中具有合适的体外特性和所需的药代动力学特性。研究了根据MTH1敲低和/或小分子抑制作用来研究DNA损伤反应,细胞活力和氧代-NTPs(氧化的核苷三磷酸)的细胞内浓度。根据我们的发现,我们无法提供证据进一步将MTH1用作肿瘤靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号