当前位置:
X-MOL 学术
›
Nat. Nanotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Positive and negative chemotaxis of enzyme-coated liposome motors.
Nature Nanotechnology ( IF 38.1 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41565-019-0578-8 Ambika Somasundar 1 , Subhadip Ghosh 2 , Farzad Mohajerani 1 , Lynnicia N Massenburg 3 , Tinglu Yang 2 , Paul S Cremer 2 , Darrell Velegol 1 , Ayusman Sen 2
Nature Nanotechnology ( IF 38.1 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41565-019-0578-8 Ambika Somasundar 1 , Subhadip Ghosh 2 , Farzad Mohajerani 1 , Lynnicia N Massenburg 3 , Tinglu Yang 2 , Paul S Cremer 2 , Darrell Velegol 1 , Ayusman Sen 2
Affiliation
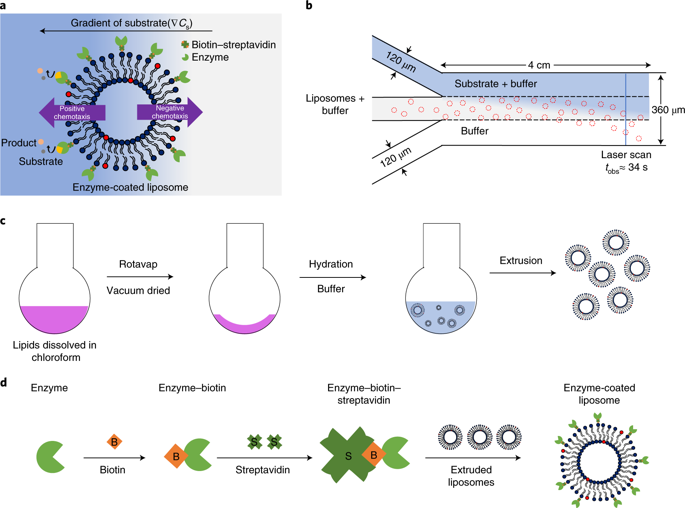
|
The ability of cells or cell components to move in response to chemical signals is critical for the survival of living systems. This motion arises from harnessing free energy from enzymatic catalysis. Artificial model protocells derived from phospholipids and other amphiphiles have been made and their enzymatic-driven motion has been observed. However, control of directionality based on chemical cues (chemotaxis) has been difficult to achieve. Here we show both positive or negative chemotaxis of liposomal protocells. The protocells move autonomously by interacting with concentration gradients of either substrates or products in enzyme catalysis, or Hofmeister salts. We hypothesize that the propulsion mechanism is based on the interplay between enzyme-catalysis-induced positive chemotaxis and solute-phospholipid-based negative chemotaxis. Controlling the extent and direction of chemotaxis holds considerable potential for designing cell mimics and delivery vehicles that can reconfigure their motion in response to environmental conditions.
中文翻译:

酶涂层脂质体马达的正趋化和负趋化作用。
细胞或细胞成分响应化学信号而移动的能力对于生命系统的生存至关重要。该运动是由于利用了来自酶催化作用的自由能而产生的。已经制备了衍生自磷脂和其他两亲物的人工模型原始细胞,并观察到它们的酶驱动运动。然而,基于化学提示(趋化性)的方向性控制已经难以实现。在这里,我们显示脂质体原细胞的正趋化或负趋化。原始细胞通过与酶催化中的底物或产物或霍夫迈斯特盐的浓度梯度相互作用来自主移动。我们假设推进机制是基于酶催化诱导的正趋化性与基于溶质磷脂的负趋化性之间的相互作用。
更新日期:2019-11-18
中文翻译:

酶涂层脂质体马达的正趋化和负趋化作用。
细胞或细胞成分响应化学信号而移动的能力对于生命系统的生存至关重要。该运动是由于利用了来自酶催化作用的自由能而产生的。已经制备了衍生自磷脂和其他两亲物的人工模型原始细胞,并观察到它们的酶驱动运动。然而,基于化学提示(趋化性)的方向性控制已经难以实现。在这里,我们显示脂质体原细胞的正趋化或负趋化。原始细胞通过与酶催化中的底物或产物或霍夫迈斯特盐的浓度梯度相互作用来自主移动。我们假设推进机制是基于酶催化诱导的正趋化性与基于溶质磷脂的负趋化性之间的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号