当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optogenetic control of cofilin and αTAT in living cells using Z-lock.
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41589-019-0405-4 Orrin J Stone 1 , Neha Pankow 1 , Bei Liu 1 , Ved P Sharma 2, 3 , Robert J Eddy 2 , Hui Wang 1 , Andrew T Putz 1 , Frank D Teets 4 , Brian Kuhlman 4 , John S Condeelis 2, 3, 5 , Klaus M Hahn 1, 6
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-11-18 , DOI: 10.1038/s41589-019-0405-4 Orrin J Stone 1 , Neha Pankow 1 , Bei Liu 1 , Ved P Sharma 2, 3 , Robert J Eddy 2 , Hui Wang 1 , Andrew T Putz 1 , Frank D Teets 4 , Brian Kuhlman 4 , John S Condeelis 2, 3, 5 , Klaus M Hahn 1, 6
Affiliation
|
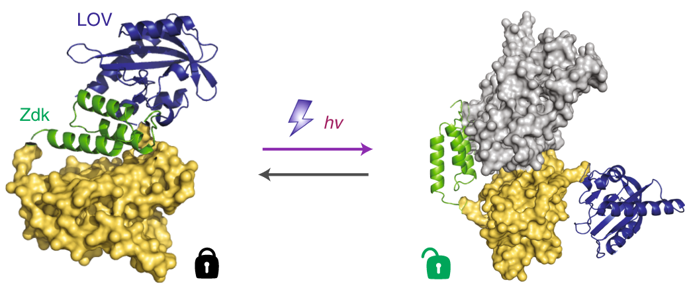
Here we introduce Z-lock, an optogenetic approach for reversible, light-controlled steric inhibition of protein active sites. The light oxygen voltage (LOV) domain and Zdk, a small protein that binds LOV selectively in the dark, are appended to the protein of interest where they sterically block the active site. Irradiation causes LOV to change conformation and release Zdk, exposing the active site. Computer-assisted protein design was used to optimize linkers and Zdk-LOV affinity, for both effective binding in the dark, and effective light-induced release of the intramolecular interaction. Z-lock cofilin was shown to have actin severing ability in vitro, and in living cancer cells it produced protrusions and invadopodia. An active fragment of the tubulin acetylase αTAT was similarly modified and shown to acetylate tubulin on irradiation.
中文翻译:

使用 Z-lock 对活细胞中的 cofilin 和 αTAT 进行光遗传学控制。
在这里,我们介绍 Z 锁,一种用于对蛋白质活性位点进行可逆、光控空间抑制的光遗传学方法。光氧电压 (LOV) 结构域和 Zdk(一种在黑暗中选择性结合 LOV 的小蛋白质)被附加到感兴趣的蛋白质上,在那里它们在空间上阻断活性位点。辐照导致 LOV 改变构象并释放 Zdk,从而暴露活性位点。计算机辅助蛋白质设计用于优化接头和 Zdk-LOV 亲和力,以实现在黑暗中的有效结合和分子内相互作用的有效光诱导释放。Z-lock cofilin 在体外被证明具有肌动蛋白切断能力,并且在活癌细胞中它产生突起和侵袭伪足。微管蛋白乙酰化酶 αTAT 的活性片段被类似地修饰并显示在照射时乙酰化微管蛋白。
更新日期:2019-11-18
中文翻译:

使用 Z-lock 对活细胞中的 cofilin 和 αTAT 进行光遗传学控制。
在这里,我们介绍 Z 锁,一种用于对蛋白质活性位点进行可逆、光控空间抑制的光遗传学方法。光氧电压 (LOV) 结构域和 Zdk(一种在黑暗中选择性结合 LOV 的小蛋白质)被附加到感兴趣的蛋白质上,在那里它们在空间上阻断活性位点。辐照导致 LOV 改变构象并释放 Zdk,从而暴露活性位点。计算机辅助蛋白质设计用于优化接头和 Zdk-LOV 亲和力,以实现在黑暗中的有效结合和分子内相互作用的有效光诱导释放。Z-lock cofilin 在体外被证明具有肌动蛋白切断能力,并且在活癌细胞中它产生突起和侵袭伪足。微管蛋白乙酰化酶 αTAT 的活性片段被类似地修饰并显示在照射时乙酰化微管蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号