当前位置:
X-MOL 学术
›
J. Appl. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards a better understanding of the release mechanisms of caffeine from PLGA microparticles
Journal of Applied Polymer Science ( IF 2.7 ) Pub Date : 2019-11-18 , DOI: 10.1002/app.48710 Fahima Tamani 1 , Mounira Chérifa Hamoudi 1 , Florence Danede 2 , Jean‐François Willart 2 , Florence Siepmann 1 , Juergen Siepmann 1
Journal of Applied Polymer Science ( IF 2.7 ) Pub Date : 2019-11-18 , DOI: 10.1002/app.48710 Fahima Tamani 1 , Mounira Chérifa Hamoudi 1 , Florence Danede 2 , Jean‐François Willart 2 , Florence Siepmann 1 , Juergen Siepmann 1
Affiliation
|
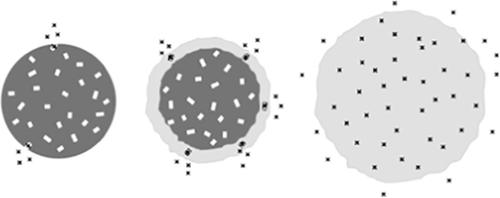
Poly(lactic‐co‐glycolic acid) (PLGA)‐based microparticles can be successfully used to control the release rate of a drug and optimize the therapeutic efficacy of a medical treatment. However, the underlying drug release mechanisms can be complex and are often not fully understood. This renders system optimization cumbersome. In this study, differently sized caffeine‐loaded PLGA microparticles were prepared and the swelling and drug release behaviors of single microparticles were monitored upon exposure to phosphate buffer pH 7.4. Ensembles of microparticles were characterized by X‐ray diffraction, differential scanning calorimetry, scanning electron microscopy, gel permeation chromatography, and optical microscopy. The observed triphasic drug release patterns could be explained as follows. The initial burst release can be attributed to the dissolution of tiny drug crystals with direct surface access. The subsequent second drug release phase (with an about constant release rate) could be attributed to the release of drug crystals in regions, which undergo local swelling. The third release phase (again rapid, leading to complete drug exhaust) could be explained by substantial polymer swelling throughout the systems. Once a critical polymer molecular weight is reached, the PLGA chains are sufficiently hydrophilic, insufficiently entangled and the osmotic pressure created by water soluble degradation products attracts high amounts of water into the system. © 2019 Wiley Periodicals, Inc. J. Appl. Polym. Sci. 2020, 137, 48710.
中文翻译:

更好地了解咖啡因从PLGA微粒中的释放机理
聚(乳酸-共基于乙醇酸(PLGA)的微粒可成功用于控制药物的释放速率并优化药物治疗的疗效。但是,潜在的药物释放机制可能很复杂,并且常常没有被完全理解。这使得系统优化麻烦。在这项研究中,制备了不同大小的咖啡因负载的PLGA微粒,并通过暴露于pH 7.4的磷酸盐缓冲液来监测单个微粒的溶胀和药物释放行为。通过X射线衍射,差示扫描量热法,扫描电子显微镜,凝胶渗透色谱和光学显微镜对微粒集合进行了表征。所观察到的三相药物释放模式可以解释如下。最初的突发释放可归因于直接进入表面的微小药物晶体的溶解。随后的第二个药物释放阶段(具有大致恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 随后的第二个药物释放阶段(大约恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 随后的第二个药物释放阶段(具有大致恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学2020,137,48710。
更新日期:2020-03-26
中文翻译:

更好地了解咖啡因从PLGA微粒中的释放机理
聚(乳酸-共基于乙醇酸(PLGA)的微粒可成功用于控制药物的释放速率并优化药物治疗的疗效。但是,潜在的药物释放机制可能很复杂,并且常常没有被完全理解。这使得系统优化麻烦。在这项研究中,制备了不同大小的咖啡因负载的PLGA微粒,并通过暴露于pH 7.4的磷酸盐缓冲液来监测单个微粒的溶胀和药物释放行为。通过X射线衍射,差示扫描量热法,扫描电子显微镜,凝胶渗透色谱和光学显微镜对微粒集合进行了表征。所观察到的三相药物释放模式可以解释如下。最初的突发释放可归因于直接进入表面的微小药物晶体的溶解。随后的第二个药物释放阶段(具有大致恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 随后的第二个药物释放阶段(大约恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 随后的第二个药物释放阶段(具有大致恒定的释放速率)可以归因于药物晶体在局部溶胀的区域中的释放。第三释放阶段(再次迅速,导致药物完全排出)可以通过整个系统中大量的聚合物溶胀来解释。一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学 一旦达到临界聚合物分子量,PLGA链就具有足够的亲水性,不充分的缠结,并且由水溶性降解产物产生的渗透压将大量的水吸引到系统中。分级为4 +©2019 Wiley Periodicals,Inc.J.Appl。Polym。科学2020,137,48710。











































 京公网安备 11010802027423号
京公网安备 11010802027423号