Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into of the Allosteric Activation of the LicT Antiterminator by PTS-Mediated Phosphorylation.
Structure ( IF 5.7 ) Pub Date : 2019-11-18 , DOI: 10.1016/j.str.2019.10.017 Yinshan Yang 1 , André Padilla 1 , Karine de Guillen 1 , Léa Mammri 1 , Jérome Gracy 1 , Nathalie Declerck 2 , Hélène Déméné 1
Structure ( IF 5.7 ) Pub Date : 2019-11-18 , DOI: 10.1016/j.str.2019.10.017 Yinshan Yang 1 , André Padilla 1 , Karine de Guillen 1 , Léa Mammri 1 , Jérome Gracy 1 , Nathalie Declerck 2 , Hélène Déméné 1
Affiliation
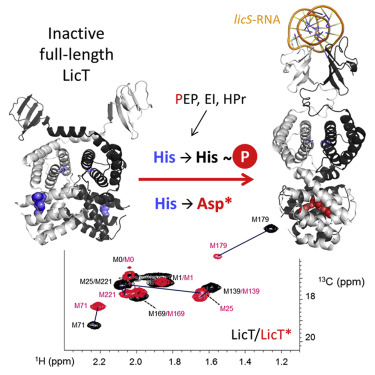
|
LicT belongs to an essential family of bacterial transcriptional antitermination proteins controlling the expression of sugar-metabolizing operons. When activated, they bind to nascent mRNAs, preventing premature arrest of transcription. The RNA binding capacity of the N-terminal domain CAT is controlled by phosphorylations of two homologous regulation modules by the phosphotransferase system (PTS). Previous studies on truncated and mutant proteins provided partial insight into the mechanism of signal transduction between the effector and regulatory modules. We report here the conformational and functional investigation on the allosteric activation of full-length LicT. Combining fluorescence anisotropy and NMR, we find a tight correlation between LicT RNA binding capacity and CAT closure upon PTS-mediated phosphorylation and phosphomimetic mutations. Our study highlights fine structural differences between activation processes. Furthermore, the NMR study of full-length proteins points to the back and forth propagation of structural restraints from the RNA binding to the distal regulatory module.
中文翻译:

通过PTS介导的磷酸化LicT抗终止剂的变构活化的结构见解。
LicT属于一个重要的细菌转录抗终止蛋白家族,可控制糖代谢操纵子的表达。激活后,它们会与新生的mRNA结合,从而防止转录的过早停止。N端结构域CAT的RNA结合能力通过两个同源调节模块的磷酸转移酶系统(PTS)的磷酸化来控制。先前对截短和突变蛋白的研究提供了对效应子和调节模块之间信号转导机制的部分了解。我们在这里报告全长LicT的变构激活的构象和功能研究。结合荧光各向异性和NMR,我们发现LicT RNA结合能力与CAT在PTS介导的磷酸化和拟磷酸酶突变后的关闭之间密切相关。我们的研究突出了激活过程之间的精细结构差异。此外,对全长蛋白质的NMR研究表明,RNA结合至远端调节模块后,结构性限制因子来回传播。
更新日期:2019-11-18
中文翻译:

通过PTS介导的磷酸化LicT抗终止剂的变构活化的结构见解。
LicT属于一个重要的细菌转录抗终止蛋白家族,可控制糖代谢操纵子的表达。激活后,它们会与新生的mRNA结合,从而防止转录的过早停止。N端结构域CAT的RNA结合能力通过两个同源调节模块的磷酸转移酶系统(PTS)的磷酸化来控制。先前对截短和突变蛋白的研究提供了对效应子和调节模块之间信号转导机制的部分了解。我们在这里报告全长LicT的变构激活的构象和功能研究。结合荧光各向异性和NMR,我们发现LicT RNA结合能力与CAT在PTS介导的磷酸化和拟磷酸酶突变后的关闭之间密切相关。我们的研究突出了激活过程之间的精细结构差异。此外,对全长蛋白质的NMR研究表明,RNA结合至远端调节模块后,结构性限制因子来回传播。



























 京公网安备 11010802027423号
京公网安备 11010802027423号