当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deficiency in the DNA repair protein ERCC1 triggers a link between senescence and apoptosis in human fibroblasts and mouse skin.
Aging Cell ( IF 8.0 ) Pub Date : 2019-11-18 , DOI: 10.1111/acel.13072 Dong Eun Kim 1 , Martijn E T Dollé 2 , Wilbert P Vermeij 3, 4 , Akos Gyenis 5 , Katharina Vogel 5 , Jan H J Hoeijmakers 3, 4, 5 , Christopher D Wiley 1 , Albert R Davalos 1 , Paul Hasty 6 , Pierre-Yves Desprez 1 , Judith Campisi 1, 7
Aging Cell ( IF 8.0 ) Pub Date : 2019-11-18 , DOI: 10.1111/acel.13072 Dong Eun Kim 1 , Martijn E T Dollé 2 , Wilbert P Vermeij 3, 4 , Akos Gyenis 5 , Katharina Vogel 5 , Jan H J Hoeijmakers 3, 4, 5 , Christopher D Wiley 1 , Albert R Davalos 1 , Paul Hasty 6 , Pierre-Yves Desprez 1 , Judith Campisi 1, 7
Affiliation
|
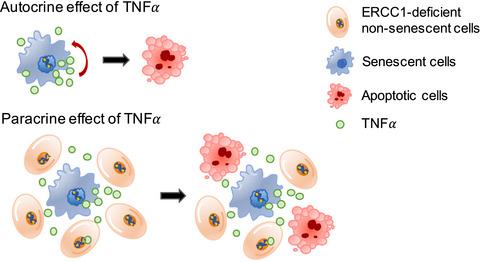
ERCC1 (excision repair cross complementing‐group 1) is a mammalian endonuclease that incises the damaged strand of DNA during nucleotide excision repair and interstrand cross‐link repair. Ercc1−/Δ mice, carrying one null and one hypomorphic Ercc1 allele, have been widely used to study aging due to accelerated aging phenotypes in numerous organs and their shortened lifespan. Ercc1−/Δ mice display combined features of human progeroid and cancer‐prone syndromes. Although several studies report cellular senescence and apoptosis associated with the premature aging of Ercc1−/Δ mice, the link between these two processes and their physiological relevance in the phenotypes of Ercc1−/Δ mice are incompletely understood. Here, we show that ERCC1 depletion, both in cultured human fibroblasts and the skin of Ercc1−/Δ mice, initially induces cellular senescence and, importantly, increased expression of several SASP (senescence‐associated secretory phenotype) factors. Cellular senescence induced by ERCC1 deficiency was dependent on activity of the p53 tumor‐suppressor protein. In turn, TNFα secreted by senescent cells induced apoptosis, not only in neighboring ERCC1‐deficient nonsenescent cells, but also cell autonomously in the senescent cells themselves. In addition, expression of the stem cell markers p63 and Lgr6 was significantly decreased in Ercc1−/Δ mouse skin, where the apoptotic cells are localized, compared to age‐matched wild‐type skin, possibly due to the apoptosis of stem cells. These data suggest that ERCC1‐depleted cells become susceptible to apoptosis via TNFα secreted from neighboring senescent cells. We speculate that parts of the premature aging phenotypes and shortened health‐ or lifespan may be due to stem cell depletion through apoptosis promoted by senescent cells.
中文翻译:

DNA修复蛋白ERCC1的缺乏触发了人类成纤维细胞和小鼠皮肤衰老与凋亡之间的联系。
ERCC1(切除修复交叉互补组1)是一种哺乳动物核酸内切酶,可在核苷酸切除修复和链间交联修复过程中切入DNA的受损链。具有一个无效和一个亚型的Ercc1等位基因的Ercc1- /Δ小鼠由于在许多器官中加速衰老表型和缩短寿命而被广泛用于研究衰老。Ercc1- /Δ小鼠表现出人类早衰和癌症易感综合症的综合特征。尽管一些研究报告了与Ercc1- /Δ小鼠过早衰老相关的细胞衰老和凋亡,但这两个过程之间的联系及其在Ercc1表型中的生理相关性-/Δ小鼠尚未完全理解。在这里,我们显示,在培养的人成纤维细胞和Ercc1- /Δ小鼠皮肤中,ERCC1的耗竭最初会诱导细胞衰老,并且重要的是,增加了几种SASP(衰老相关分泌表型)因子的表达。ERCC1缺乏引起的细胞衰老取决于p53肿瘤抑制蛋白的活性。反过来,衰老细胞分泌的TNFα不仅诱导邻近ERCC1缺陷的非衰老细胞凋亡,而且诱导衰老细胞自身自主凋亡。另外, Ercc1- /Δ中干细胞标志物p63和Lgr6的表达显着降低。与年龄匹配的野生型皮肤相比,凋亡的皮肤位于小鼠皮肤中,这可能是由于干细胞的凋亡所致。这些数据表明,耗尽ERCC1的细胞通过相邻衰老细胞分泌的TNFα变得易于凋亡。我们推测部分早衰表型和健康或寿命缩短可能是由于衰老细胞促进细胞凋亡导致干细胞耗竭所致。
更新日期:2019-11-18
中文翻译:

DNA修复蛋白ERCC1的缺乏触发了人类成纤维细胞和小鼠皮肤衰老与凋亡之间的联系。
ERCC1(切除修复交叉互补组1)是一种哺乳动物核酸内切酶,可在核苷酸切除修复和链间交联修复过程中切入DNA的受损链。具有一个无效和一个亚型的Ercc1等位基因的Ercc1- /Δ小鼠由于在许多器官中加速衰老表型和缩短寿命而被广泛用于研究衰老。Ercc1- /Δ小鼠表现出人类早衰和癌症易感综合症的综合特征。尽管一些研究报告了与Ercc1- /Δ小鼠过早衰老相关的细胞衰老和凋亡,但这两个过程之间的联系及其在Ercc1表型中的生理相关性-/Δ小鼠尚未完全理解。在这里,我们显示,在培养的人成纤维细胞和Ercc1- /Δ小鼠皮肤中,ERCC1的耗竭最初会诱导细胞衰老,并且重要的是,增加了几种SASP(衰老相关分泌表型)因子的表达。ERCC1缺乏引起的细胞衰老取决于p53肿瘤抑制蛋白的活性。反过来,衰老细胞分泌的TNFα不仅诱导邻近ERCC1缺陷的非衰老细胞凋亡,而且诱导衰老细胞自身自主凋亡。另外, Ercc1- /Δ中干细胞标志物p63和Lgr6的表达显着降低。与年龄匹配的野生型皮肤相比,凋亡的皮肤位于小鼠皮肤中,这可能是由于干细胞的凋亡所致。这些数据表明,耗尽ERCC1的细胞通过相邻衰老细胞分泌的TNFα变得易于凋亡。我们推测部分早衰表型和健康或寿命缩短可能是由于衰老细胞促进细胞凋亡导致干细胞耗竭所致。









































 京公网安备 11010802027423号
京公网安备 11010802027423号