当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting Bacterial Sortase A with Covalent Inhibitors: 27 New Starting Points for Structure-Based Hit-to-Lead Optimization.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-18 , DOI: 10.1021/acsinfecdis.9b00265 Kristaps Jaudzems 1, 2 , Viktorija Kurbatska 3 , Atis Je Kabsons 1 , Raitis Bobrovs 1 , Zhanna Rudevica 3 , Ainars Leonchiks 3
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-18 , DOI: 10.1021/acsinfecdis.9b00265 Kristaps Jaudzems 1, 2 , Viktorija Kurbatska 3 , Atis Je Kabsons 1 , Raitis Bobrovs 1 , Zhanna Rudevica 3 , Ainars Leonchiks 3
Affiliation
|
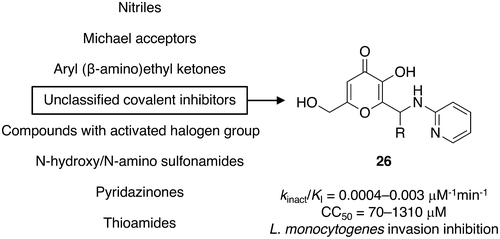
Because of its essential role as a bacterial virulence factor, enzyme sortase A (SrtA) has become an attractive target for the development of new antivirulence drugs against Gram-positive infections. Here we describe 27 compounds identified as covalent inhibitors of Staphylococcus aureus SrtA by screening a library of approximately 50 000 compounds using a FRET assay followed by NMR-based validation and binding reversibility analysis. Nineteen of these compounds displayed only moderate to weak cytotoxicity, with CC50 against NIH 3T3 mice fibroblast cells ranging from 12 to 740 μM. Analysis using covalent docking suggests that the inhibitors initially associate via hydrophobic interactions, followed by covalent bond formation between the SrtA active site cysteine and an electrophilic center of the inhibitor. The compounds represent good starting points that have the potential to be developed into broad spectrum antivirulence agents as exemplified by hit-to-lead optimization of one of the compounds.
中文翻译:

使用共价抑制剂靶向细菌分选酶A:基于结构的按铅优化的27个新起点。
由于其作为细菌毒力因子的重要作用,酶分选酶A(SrtA)已成为开发针对革兰氏阳性感染的新型抗毒药物的有吸引力的目标。在这里,我们通过使用FRET分析筛选大约50,000种化合物的库,然后进行基于NMR的验证和结合可逆性分析,来描述27种被鉴定为金黄色葡萄球菌SrtA的共价抑制剂的化合物。这些化合物中有19种仅表现出中度至弱细胞毒性,针对NIH 3T3小鼠成纤维细胞的CC50为12至740μM。使用共价对接的分析表明,抑制剂最初是通过疏水相互作用缔合的,然后在SrtA活性位点半胱氨酸和抑制剂的亲电子中心之间形成共价键。
更新日期:2019-11-18
中文翻译:

使用共价抑制剂靶向细菌分选酶A:基于结构的按铅优化的27个新起点。
由于其作为细菌毒力因子的重要作用,酶分选酶A(SrtA)已成为开发针对革兰氏阳性感染的新型抗毒药物的有吸引力的目标。在这里,我们通过使用FRET分析筛选大约50,000种化合物的库,然后进行基于NMR的验证和结合可逆性分析,来描述27种被鉴定为金黄色葡萄球菌SrtA的共价抑制剂的化合物。这些化合物中有19种仅表现出中度至弱细胞毒性,针对NIH 3T3小鼠成纤维细胞的CC50为12至740μM。使用共价对接的分析表明,抑制剂最初是通过疏水相互作用缔合的,然后在SrtA活性位点半胱氨酸和抑制剂的亲电子中心之间形成共价键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号