当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted cysteine scanning in D1-S6 of the sodium channel hNav1.4 alters kinetics and structural interactions of slow inactivation.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-11-15 , DOI: 10.1016/j.bbamem.2019.183129 Jonathan M Beard 1 , Penny E Shockett 1 , John P O'Reilly 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-11-15 , DOI: 10.1016/j.bbamem.2019.183129 Jonathan M Beard 1 , Penny E Shockett 1 , John P O'Reilly 1
Affiliation
|
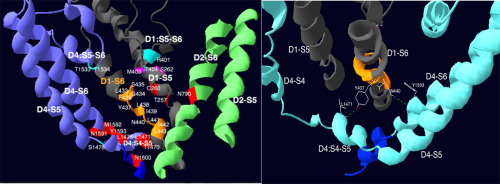
Slow inactivation in voltage-gated Na+ channels (Navs) plays an important physiological role in excitable tissues (muscle, heart, nerves) and mutations that disrupt Nav slow inactivation can result in pathophysiologies (myotonia, arrhythmias, epilepsy). While the molecular mechanisms responsible for slow inactivation remain elusive, previous studies have suggested a role for the pore-lining D1-S6 helix. The goals of this research were to determine if (1) cysteine substitutions in D1-S6 affect gating kinetics and (2) methanethiosulfonate ethylammonium (MTSEA) accessibility changes in different kinetic states. Site-directed mutagenesis in the human skeletal muscle isoform hNav1.4 was used to substitute cysteine for eleven amino acids in D1-S6 from L433 to L443. Mutants were expressed in HEK cells and recorded from with whole-cell patch clamp. All mutations affected one or more baseline kinetics of the sodium channel, including activation, fast inactivation, and slow inactivation. Substitution of cysteine (for nonpolar residues) adjacent to polar residues destabilized slow inactivation in G434C, F436C, I439C, and L441C. Cysteine substitution without adjacent polar residues enhanced slow inactivation in L438C and N440C, and disrupted possible H-bonds involving Y437:D4 S4-S5 and N440:D4-S6. MTSEA exposure in closed, fast-inactivated, or slow-inactivated states in most mutants had little-to-no effect. In I439C, MTSEA application in closed, fast-inactivated, and slow-inactivated states produced irreversible reduction in current, suggesting I439C accessibility to MTSEA in all three kinetic states. D1-S6 is important for Nav gating kinetics, stability of slow-inactivated state, structural contacts, and state-dependent positioning. However, prominent reconfiguration of D1-S6 may not occur in slow inactivation.
中文翻译:

钠通道hNav1.4的D1-S6中取代的半胱氨酸扫描可改变慢灭活的动力学和结构相互作用。
电压门控Na +通道(Navs)中的缓慢失活在可兴奋组织(肌肉,心脏,神经)中起重要的生理作用,破坏Nav缓慢失活的突变会导致病理生理(肌强直,心律不齐,癫痫病)。虽然导致缓慢失活的分子机制仍然难以捉摸,但先前的研究表明,这种孔衬D1-S6螺旋的作用。这项研究的目的是确定(1)D1-S6中的半胱氨酸取代是否影响选通动力学和(2)甲硫代磺酸盐乙铵(MTSEA)在不同动力学状态下的可及性变化。人类骨骼肌同工型hNav1.4中的定点诱变用于从L433到L443中用半胱氨酸替代D1-S6中的11个氨基酸。突变体在HEK细胞中表达,并用全细胞膜片钳记录。所有突变都会影响钠通道的一种或多种基线动力学,包括激活,快速失活和缓慢失活。在G434C,F436C,I439C和L441C中,与极性残基相邻的半胱氨酸取代(对于非极性残基)使慢速失活不稳定。没有相邻极性残基的半胱氨酸取代增强了L438C和N440C的缓慢灭活,并破坏了涉及Y437:D4 S4-S5和N440:D4-S6的可能的H键。在大多数突变体中,处于闭合,快速灭活或缓慢灭活状态的MTSEA暴露几乎没有影响。在I439C中,在闭合,快速灭活和缓慢灭活状态下应用MTSEA会产生不可逆的电流减少,这表明I439C在所有三种动力学状态下都可接近MTSEA。D1-S6对于导航门控动力学,慢灭活状态的稳定性,结构性接触,以及与状态有关的定位。但是,在缓慢的灭活过程中可能不会发生D1-S6的显着重新配置。
更新日期:2019-11-18
中文翻译:

钠通道hNav1.4的D1-S6中取代的半胱氨酸扫描可改变慢灭活的动力学和结构相互作用。
电压门控Na +通道(Navs)中的缓慢失活在可兴奋组织(肌肉,心脏,神经)中起重要的生理作用,破坏Nav缓慢失活的突变会导致病理生理(肌强直,心律不齐,癫痫病)。虽然导致缓慢失活的分子机制仍然难以捉摸,但先前的研究表明,这种孔衬D1-S6螺旋的作用。这项研究的目的是确定(1)D1-S6中的半胱氨酸取代是否影响选通动力学和(2)甲硫代磺酸盐乙铵(MTSEA)在不同动力学状态下的可及性变化。人类骨骼肌同工型hNav1.4中的定点诱变用于从L433到L443中用半胱氨酸替代D1-S6中的11个氨基酸。突变体在HEK细胞中表达,并用全细胞膜片钳记录。所有突变都会影响钠通道的一种或多种基线动力学,包括激活,快速失活和缓慢失活。在G434C,F436C,I439C和L441C中,与极性残基相邻的半胱氨酸取代(对于非极性残基)使慢速失活不稳定。没有相邻极性残基的半胱氨酸取代增强了L438C和N440C的缓慢灭活,并破坏了涉及Y437:D4 S4-S5和N440:D4-S6的可能的H键。在大多数突变体中,处于闭合,快速灭活或缓慢灭活状态的MTSEA暴露几乎没有影响。在I439C中,在闭合,快速灭活和缓慢灭活状态下应用MTSEA会产生不可逆的电流减少,这表明I439C在所有三种动力学状态下都可接近MTSEA。D1-S6对于导航门控动力学,慢灭活状态的稳定性,结构性接触,以及与状态有关的定位。但是,在缓慢的灭活过程中可能不会发生D1-S6的显着重新配置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号