当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosimilars in Developed Economies: Overview, Status, and Regulatory Considerations.
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.yrtph.2019.104525 Anurag S Rathore 1 , Ankita Bhargava 1
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2019-11-16 , DOI: 10.1016/j.yrtph.2019.104525 Anurag S Rathore 1 , Ankita Bhargava 1
Affiliation
|
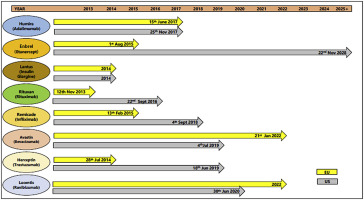
Biotherapeutics dominate the pipelines of pharmaceutical companies across the world today with products ranging from hormones, immune-modulators, monoclonal antibodies (mAbs), blood coagulation factors, enzymes and vaccines. However, they are considerably more expensive than their small molecule counterparts (pharmaceuticals) and as a result their contribution to the already unacceptably high healthcare costs in the developed economies (Europe, United States, Japan, Canada and South Korea) has been in the limelight in the last decade. This has resulted in the rise of biosimilars, seen as the affordable versions of innovator biotherapeutics. As the developed economies form the majority of the global sales of biotherapeutics, they are an attractive market for the biosimilars as well. Regulatory considerations for approval of biosimilars in these jurisdictions is likely to have a major impact on the adoption of biosimilars. In this paper, we offer a perspective on this topic while focusing on the developed markets. This article summarizes the main regulatory requirements for approval of biosimilars in Europe, United States, Japan, Canada, and South Korea. An overview on current biosimilars status and market in the aforesaid countries has also been included.
中文翻译:

发达经济体中的生物仿制药:概述,现状和监管注意事项。
生物治疗药物在激素,免疫调节剂,单克隆抗体(mAb),凝血因子,酶和疫苗等产品中占据着全球制药公司的主导地位。但是,它们比小分子同类产品(药品)贵得多,因此,它们对发达经济体(欧洲,美国,日本,加拿大和韩国)本已令人无法接受的高昂医疗费用做出了贡献在过去的十年中。这导致了生物仿制药的兴起,被视为创新型生物疗法的价格可承受的版本。由于发达经济体占生物治疗剂全球销售的大部分,因此它们也是生物仿制药的诱人市场。在这些司法管辖区批准生物仿制药的监管考虑可能会对生物仿制药的采用产生重大影响。在本文中,我们在关注发达市场的同时提供了关于该主题的观点。本文总结了欧洲,美国,日本,加拿大和韩国批准生物仿制药的主要法规要求。还包括对上述国家当前生物仿制药的现状和市场的概述。
更新日期:2019-11-18
中文翻译:

发达经济体中的生物仿制药:概述,现状和监管注意事项。
生物治疗药物在激素,免疫调节剂,单克隆抗体(mAb),凝血因子,酶和疫苗等产品中占据着全球制药公司的主导地位。但是,它们比小分子同类产品(药品)贵得多,因此,它们对发达经济体(欧洲,美国,日本,加拿大和韩国)本已令人无法接受的高昂医疗费用做出了贡献在过去的十年中。这导致了生物仿制药的兴起,被视为创新型生物疗法的价格可承受的版本。由于发达经济体占生物治疗剂全球销售的大部分,因此它们也是生物仿制药的诱人市场。在这些司法管辖区批准生物仿制药的监管考虑可能会对生物仿制药的采用产生重大影响。在本文中,我们在关注发达市场的同时提供了关于该主题的观点。本文总结了欧洲,美国,日本,加拿大和韩国批准生物仿制药的主要法规要求。还包括对上述国家当前生物仿制药的现状和市场的概述。











































 京公网安备 11010802027423号
京公网安备 11010802027423号