Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.40066 Jie Yang 1 , Dihua Dai 1 , Xinyue Lou 1 , Lianjun Ma 1 , Bailiang Wang 2 , Ying-Wei Yang 1, 3
|
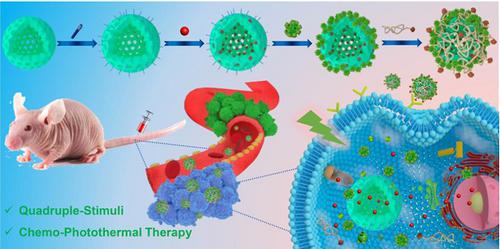
Multifunctional supramolecular nanoplatforms that integrate the advantages of different therapeutic techniques can trigger multimodal synergistic treatment of tumors, thus representing an emerging powerful tool for cancer therapeutics.
Methods: In this work, we design and fabricate a multifunctional supramolecular drug delivery platform, namely Fa-mPEG@CP5-CuS@HMSN-Py nanoparticles (FaPCH NPs), consisting of a pyridinium (Py)-modified hollow mesoporous silica nanoparticles-based drug reservoir (HMSN-Py) with high loading capacity, a layer of NIR-operable carboxylatopillar[5]arene (CP5)-functionalized CuS nanoparticles (CP5-CuS) on the surface of HMSN-Py connected through supramolecular host-guest interactions between CP5 rings and Py stalks, and another layer of folic acid (Fa)-conjugated polyethylene glycol (Fa-PEG) antennas by electrostatic interactions capable of active targeting at tumor lesions, in a controlled, highly integrated fashion for synergistic chemo-photothermal therapy.
Results: Fa-mPEG antennas endowed the enhanced active targeting effect toward cancer cells, and CP5-CuS served as not only a quadruple-stimuli responsive nanogate for controllable drug release but also a special agent for NIR-guided photothermal therapy. Meanwhile, anticancer drug doxorubicin (DOX) could be released from the HMSN-Py reservoirs under tumor microenvironments for chemotherapy, thus realizing multimodal synergistic therapeutics. Such a supramolecular drug delivery platform showed effective synergistic chemo-photothermal therapy both in vitro and in vivo.
Conclusion: This novel supramolecular nanoplatform possesses great potential in controlled drug delivery and tumor cellular internalization for synergistic chemo-photothermal therapy, providing a promising approach for multimodal synergistic cancer treatment.
中文翻译:

基于中空介孔药物载体和大环封端的 CuS 纳米门的超分子纳米材料,用于协同化学光热治疗。
多功能超分子纳米平台整合了不同治疗技术的优点,可以引发肿瘤的多模式协同治疗,从而成为癌症治疗的新兴强大工具。
方法:在这项工作中,我们设计并制造了一个多功能超分子药物递送平台,即Fa-mPEG@CP5-CuS@HMSN-Py纳米颗粒(FaPCH NPs),由吡啶(Py)修饰的中空介孔二氧化硅纳米颗粒组成。具有高负载能力的药物储库(HMSN-Py),HMSN-Py表面上有一层近红外可操作的羧基柱[5]芳烃(CP5)功能化的CuS纳米颗粒(CP5-CuS),通过超分子主客体相互作用连接CP5环和Py茎,以及另一层叶酸(Fa)共轭聚乙二醇(Fa-PEG)天线,通过静电相互作用能够主动靶向肿瘤病变,以受控、高度集成的方式进行协同化学光热治疗。
结果:Fa-mPEG天线对癌细胞具有增强的主动靶向作用,CP5-CuS不仅可以作为可控药物释放的四重刺激响应纳米门,而且可以作为近红外引导光热治疗的特殊剂。同时,抗癌药物阿霉素(DOX)可以从肿瘤微环境下的HMSN-Py库中释放出来进行化疗,从而实现多模式协同治疗。这种超分子药物递送平台在体外和体内均显示出有效的协同化学光热疗法。
结论:这种新型超分子纳米平台在协同化学光热疗法的受控药物递送和肿瘤细胞内化方面具有巨大潜力,为多模式协同癌症治疗提供了一种有前途的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号