当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolism of Benzalkonium Chlorides by Human Hepatic Cytochromes P450.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.chemrestox.9b00293 Ryan P Seguin 1 , Josi M Herron 2 , Vanessa A Lopez 1 , Joseph L Dempsey 2 , Libin Xu 1, 2
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.chemrestox.9b00293 Ryan P Seguin 1 , Josi M Herron 2 , Vanessa A Lopez 1 , Joseph L Dempsey 2 , Libin Xu 1, 2
Affiliation
|
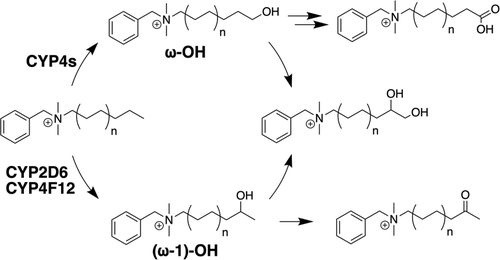
Benzalkonium chlorides (BACs) are widely used as disinfectants in cleaning products, medical products, and the food processing industry. Despite a wide range of reported toxicities, limited studies have been conducted on the metabolism of these compounds in animal models and none in human-derived cells or tissues. In this work, we report on the metabolism of BACs in human liver microsomes (HLM) and by recombinant human hepatic cytochrome P450 (CYP) enzymes. BAC metabolism in HLM was NADPH-dependent and displayed apparent half-lives that increased with BAC alkyl chain length (C10 < C12 < C14 < C16), suggesting enhanced metabolic stability of the more lipophilic, longer chain BACs. Metabolites of d7-benzyl labeled BAC substrates retained all deuteriums and there was no evidence of N-dealkylation. Tandem mass spectrometry fragmentation of BAC metabolites confirmed that oxidation occurs on the alkyl chain region. Major metabolites of C10-BAC were identified as ω-hydroxy-, (ω-1)-hydroxy-, (ω, ω-1)-diol-, (ω-1)-ketone-, and ω-carboxylic acid-C10-BAC by liquid chromatography-mass spectrometry comparison with synthetic standards. In a screen of hepatic CYP isoforms, recombinant CYP2D6, CYP4F2, and CYP4F12 consumed substantial quantities of BAC substrates and produced the major microsomal metabolites. The use of potent pan-CYP4 inhibitor HET0016, the specific CYP2D6 inhibitor quinidine, or both confirmed major contributions of CYP4- and CYP2D6-mediated metabolism in the microsomal disappearance of BACs. Kinetic characterization of C10-BAC metabolite formation in HLM demonstrated robust Michaelis-Menten kinetic parameters for ω-hydroxylation (Vmax = 380 pmol/min/mg, Km = 0.69 μM) and (ω-1)-hydroxylation (Vmax = 126 pmol/min/mg, Km = 0.13 μM) reactions. This work illustrates important roles for CYP4-mediated ω-hydroxylation and CYP2D6/CYP4-mediated (ω-1)-hydroxylation during the hepatic elimination of BACs, an environmental contaminant of emerging concern. Furthermore, we demonstrate that CYP-mediated oxidation of C10-BAC mitigates the potent inhibition of cholesterol biosynthesis exhibited by this short-chain BAC.
中文翻译:

人肝细胞色素 P450 对苯扎氯铵的代谢。
苯扎氯铵 (BAC) 广泛用作清洁产品、医疗产品和食品加工行业的消毒剂。尽管报道了多种毒性,但在动物模型中对这些化合物的代谢进行了有限的研究,而在人源细胞或组织中却没有进行任何研究。在这项工作中,我们报告了 BAC 在人肝微粒体 (HLM) 中以及通过重组人肝细胞色素 P450 (CYP) 酶的代谢。 HLM 中的 BAC 代谢依赖于 NADPH,并且表现出明显的半衰期,该半衰期随着 BAC 烷基链长度 (C10 < C12 < C14 < C16) 的增加而增加,这表明亲脂性更强、链更长的 BAC 的代谢稳定性增强。 d7-苄基标记的 BAC 底物的代谢物保留了所有氘,并且没有 N-脱烷基化的证据。 BAC 代谢物的串联质谱裂解证实烷基链区域发生氧化。 C10-BAC的主要代谢物被鉴定为ω-羟基-、(ω-1)-羟基-、(ω, ω-1)-二醇-、(ω-1)-酮-和ω-羧酸-C10 -BAC 通过液相色谱-质谱法与合成标准品进行比较。在肝 CYP 亚型筛选中,重组 CYP2D6、CYP4F2 和 CYP4F12 消耗大量 BAC 底物并产生主要微粒体代谢物。强效泛 CYP4 抑制剂 HET0016、特异性 CYP2D6 抑制剂奎尼丁或两者的使用证实了 CYP4 和 CYP2D6 介导的代谢在 BAC 微粒体消失中的主要贡献。 HLM 中 C10-BAC 代谢物形成的动力学表征证明了 ω-羟基化(Vmax = 380 pmol/min/mg,Km = 0.69 μM)和 (ω-1)-羟基化(Vmax = 126 pmol/)的稳健 Michaelis-Menten 动力学参数分钟/毫克,Km = 0.13 μM)反应。 这项工作阐明了 CYP4 介导的 ω-羟基化和 CYP2D6/CYP4 介导的 (ω-1)-羟基化在肝脏消除 BAC(一种新出现的环境污染物)过程中的重要作用。此外,我们证明 CYP 介导的 C10-BAC 氧化减轻了这种短链 BAC 对胆固醇生物合成的有效抑制。
更新日期:2019-12-04
中文翻译:

人肝细胞色素 P450 对苯扎氯铵的代谢。
苯扎氯铵 (BAC) 广泛用作清洁产品、医疗产品和食品加工行业的消毒剂。尽管报道了多种毒性,但在动物模型中对这些化合物的代谢进行了有限的研究,而在人源细胞或组织中却没有进行任何研究。在这项工作中,我们报告了 BAC 在人肝微粒体 (HLM) 中以及通过重组人肝细胞色素 P450 (CYP) 酶的代谢。 HLM 中的 BAC 代谢依赖于 NADPH,并且表现出明显的半衰期,该半衰期随着 BAC 烷基链长度 (C10 < C12 < C14 < C16) 的增加而增加,这表明亲脂性更强、链更长的 BAC 的代谢稳定性增强。 d7-苄基标记的 BAC 底物的代谢物保留了所有氘,并且没有 N-脱烷基化的证据。 BAC 代谢物的串联质谱裂解证实烷基链区域发生氧化。 C10-BAC的主要代谢物被鉴定为ω-羟基-、(ω-1)-羟基-、(ω, ω-1)-二醇-、(ω-1)-酮-和ω-羧酸-C10 -BAC 通过液相色谱-质谱法与合成标准品进行比较。在肝 CYP 亚型筛选中,重组 CYP2D6、CYP4F2 和 CYP4F12 消耗大量 BAC 底物并产生主要微粒体代谢物。强效泛 CYP4 抑制剂 HET0016、特异性 CYP2D6 抑制剂奎尼丁或两者的使用证实了 CYP4 和 CYP2D6 介导的代谢在 BAC 微粒体消失中的主要贡献。 HLM 中 C10-BAC 代谢物形成的动力学表征证明了 ω-羟基化(Vmax = 380 pmol/min/mg,Km = 0.69 μM)和 (ω-1)-羟基化(Vmax = 126 pmol/)的稳健 Michaelis-Menten 动力学参数分钟/毫克,Km = 0.13 μM)反应。 这项工作阐明了 CYP4 介导的 ω-羟基化和 CYP2D6/CYP4 介导的 (ω-1)-羟基化在肝脏消除 BAC(一种新出现的环境污染物)过程中的重要作用。此外,我们证明 CYP 介导的 C10-BAC 氧化减轻了这种短链 BAC 对胆固醇生物合成的有效抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号