当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Halofunctionalized Indenes via a Cascade Prins‐Nazarov Cyclization Promoted by Dual Roles of BX3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901266 Sabera Sultana 1 , Yong Rok Lee 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1002/adsc.201901266 Sabera Sultana 1 , Yong Rok Lee 1
Affiliation
|
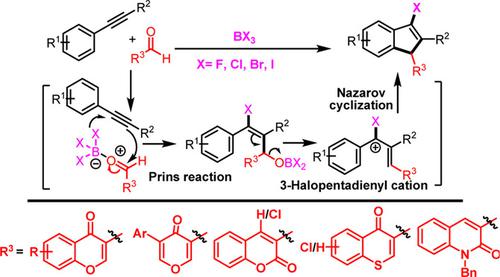
Halofunctionalization of various unactivated arylalkynes to the corresponding 1H‐indenes in the presence of a particular class of carboxaldehydes and boron trihalides (BX3, X=F, Cl, Br, I) is described. A diverse array of halofunctionalized indenes substituted with a heterocycle has been synthesized regioselectively with BX3 as a promotor for the carbocyclization and a source of X− for halogenation. This reaction proceeds via a formal halogenative [4+1] cycloaddition between arylalkynes and carboxaldehydes promoted by boron trihalides to generate halofunctionalized indenes. The usefulness of the halofunctionalized indenes was demonstrated by their conversion to other derivatives via coupling, nucleophilic substitution, and oxidation.
中文翻译:

通过BX3双重作用促进的级联Prins-Nazarov环化反应构建卤代官能化的茚。
描述了在特定类别的甲醛和三卤化硼(BX 3,X = F,Cl,Br,I)存在下,各种未活化的芳基炔烃向相应的1H-茚进行卤官能化的过程。用杂环取代的halofunctionalized茚的多样化已经BX区域选择性合成的3作为carbocyclization一个启动子和X的源-用于卤化。该反应通过由三卤化硼促进的芳基炔烃和甲醛之间的正式卤代[4 + 1]环加成反应进行,以生成卤代官能化的茚。卤代官能化的茚的有用性通过它们通过偶联,亲核取代和氧化转化为其他衍生物来证明。
更新日期:2020-01-17
中文翻译:

通过BX3双重作用促进的级联Prins-Nazarov环化反应构建卤代官能化的茚。
描述了在特定类别的甲醛和三卤化硼(BX 3,X = F,Cl,Br,I)存在下,各种未活化的芳基炔烃向相应的1H-茚进行卤官能化的过程。用杂环取代的halofunctionalized茚的多样化已经BX区域选择性合成的3作为carbocyclization一个启动子和X的源-用于卤化。该反应通过由三卤化硼促进的芳基炔烃和甲醛之间的正式卤代[4 + 1]环加成反应进行,以生成卤代官能化的茚。卤代官能化的茚的有用性通过它们通过偶联,亲核取代和氧化转化为其他衍生物来证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号