当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzyme and AIEgens Modulated Solid‐State Nanochannels: In Situ and Noninvasive Monitoring of H2O2 Released from Living Cells
Small Methods ( IF 10.7 ) Pub Date : 2019-11-15 , DOI: 10.1002/smtd.201900432 Xiaoding Lou 1, 2 , Yongjun Song 1 , Rui Liu 1 , Yong Cheng 3 , Jun Dai 3 , Qing Chen 1 , Pengcheng Gao 1 , Zujin Zhao 4 , Fan Xia 1
Small Methods ( IF 10.7 ) Pub Date : 2019-11-15 , DOI: 10.1002/smtd.201900432 Xiaoding Lou 1, 2 , Yongjun Song 1 , Rui Liu 1 , Yong Cheng 3 , Jun Dai 3 , Qing Chen 1 , Pengcheng Gao 1 , Zujin Zhao 4 , Fan Xia 1
Affiliation
|
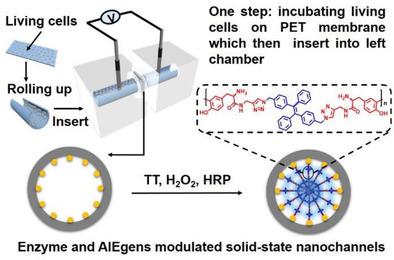
Solid‐state nanochannels have revealed great abilities in the sensing of ions, small biomolecules, and biological macromolecules. However, the current platform requires pretreatment of real samples to extract targets, which may induce false signals due to complicated collection and addition processes. Although nanopore electrodes or nanopipettes have been successfully utilized in the detection of intracellular redox‐active species without sample preparation processes, the insertion of nanoprobes to living cells is inevitable. Here, a strategy is reported to monitor H2O2 released from living cells based on functionalized solid‐state nanochannels without an insertion procedure. In this strategy, aggregation‐induced emission luminogens (AIEgens) with enzyme‐responsive linkage properties are combined in solid‐state nanochannels. When H2O2 released from cervical cancer cells (HeLa), Tyr‐containing AIEgens (TT) will form horseradish peroxidase‐modulated (HRP‐modulated) linkages in the nanochannels. The formation of linkages can result in the effective blockade of nanochannels, hence via transmembrane ionic current. Owing to the aggregation of linkage products, a fluorescence signal can be observed. By using these dual‐signal‐output nanochannels, in situ and noninvasive detection of H2O2 is able to be achieved. Together with molecular dynamic (MD) simulations results, solid surface zeta potential and contact angle experiments, it is concluded that the blockage of nanochannels is the dominate factor for ionic currents as well as fluorescence change.
中文翻译:

酶和AIEgens调节的固态纳米通道:从活细胞释放的H2O2的原位和无创监测
固态纳米通道在离子,小生物分子和生物大分子的传感方面显示出了强大的能力。但是,当前平台需要对真实样本进行预处理以提取目标,由于复杂的收集和添加过程,这可能会诱发错误信号。尽管无需样品制备过程,纳米孔电极或纳米移液器已成功用于细胞内氧化还原活性物质的检测,但将纳米探针插入活细胞是不可避免的。在此,报告了一种监视H 2 O 2的策略无需插入程序即可从功能化固态纳米通道的活细胞中释放出来。在此策略中,具有酶反应性连锁特性的聚集诱导发射发光剂(AIEgens)结合在固态纳米通道中。当H 2 ö 2从子宫颈癌细胞(HeLa细胞),含酪氨酸- AIEgens(TT)将形成在纳米通道辣根过氧化物酶调制(HRP调制)连接释放。键的形成可以导致纳米通道的有效阻断,因此可以通过跨膜离子电流。由于键合产物的聚集,可以观察到荧光信号。通过使用这些双信号输出纳米通道,原位和无创检测H 2 O 2是可以实现的。结合分子动力学(MD)模拟结果,固体表面zeta电位和接触角实验,可以得出结论,纳米通道的阻塞是离子电流以及荧光变化的主要因素。
更新日期:2019-11-15
中文翻译:

酶和AIEgens调节的固态纳米通道:从活细胞释放的H2O2的原位和无创监测
固态纳米通道在离子,小生物分子和生物大分子的传感方面显示出了强大的能力。但是,当前平台需要对真实样本进行预处理以提取目标,由于复杂的收集和添加过程,这可能会诱发错误信号。尽管无需样品制备过程,纳米孔电极或纳米移液器已成功用于细胞内氧化还原活性物质的检测,但将纳米探针插入活细胞是不可避免的。在此,报告了一种监视H 2 O 2的策略无需插入程序即可从功能化固态纳米通道的活细胞中释放出来。在此策略中,具有酶反应性连锁特性的聚集诱导发射发光剂(AIEgens)结合在固态纳米通道中。当H 2 ö 2从子宫颈癌细胞(HeLa细胞),含酪氨酸- AIEgens(TT)将形成在纳米通道辣根过氧化物酶调制(HRP调制)连接释放。键的形成可以导致纳米通道的有效阻断,因此可以通过跨膜离子电流。由于键合产物的聚集,可以观察到荧光信号。通过使用这些双信号输出纳米通道,原位和无创检测H 2 O 2是可以实现的。结合分子动力学(MD)模拟结果,固体表面zeta电位和接触角实验,可以得出结论,纳米通道的阻塞是离子电流以及荧光变化的主要因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号