Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study.
The Lancet ( IF 98.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/s0140-6736(19)32556-5 Michel Attal , Paul G Richardson , S Vincent Rajkumar , Jesus San-Miguel , Meral Beksac , Ivan Spicka , Xavier Leleu , Fredrik Schjesvold , Philippe Moreau , Meletios A Dimopoulos , Jeffrey Shang-Yi Huang , Jiri Minarik , Michele Cavo , H Miles Prince , Sandrine Macé , Kathryn P Corzo , Frank Campana , Solenn Le-Guennec , Franck Dubin , Kenneth C Anderson , Michel Attal , Paul G. Richardson , Vincent Rajkumar , Jesus San-Miguel , Meral Beksac , Ivan Spicka , Xavier Leleu , Fredrik Schjesvold , Philippe Moreau , Meletios A. Dimopoulos , Jeffrey Shang-Yi Huang , Jiri Minarik , Michele Cavo , H. Miles Prince , Sandrine Macé , Kathryn P. Corzo , Frank Campana , Solenn Le-Guennec , Franck Dubin , Kenneth C. Anderson , Simon Harrison , Wojt Janowski , Ian Kerridge , Andrew Spencer , Michel Delforge , Karel Fostier , Philip Vlummens , Ka Lung Wu , Richard Leblanc , Michel Pavic , Michael Sebag , Roman Hajek , Vladimir Maisnar , Ludek Pour , Henrik Gregersen , Lotfi Benbouker , Denis Caillot , Martine Escoffre-Barbe , Thierry Facon , Laurent Frenzel , Cyrille Hulin , Lionel Karlin , Brigitte Kolb , Brigitte Pegourie , Aurore Perrot , Mourad Tiab , Laure Vincent , Dietger Niederwieser , Achilles Anagnostopoulos , Sosana Delimpasi , Marie-Christine Kyrtsonis , Anargyros Symeonidis , Arpad Illes , Gabor Mikala , Zsolt Nagy , Sara Bringen , Paolo Corradini , Ciceri Fabio , Roberto Lemoli , Anna Liberati , Chiara Nozzoli , Renato Zambello , Shinsuke Iida , Takashi Ikeda , Satoshi Iyama , Morio Matsumoto , Chihiro Shimazaki , Kazutaka Sunami , Kenshi Suzuki , Michihiro Uchiyama , Youngil Koh , Kihyun Kim , Jae Hoon Lee , Chang-Ki Min , Hillary Blacklock , Hugh Goodman , Annette Neylon , David Simpson , Sebastian Grosicki , Artur Jurczyszyn , Adam Walter-Croneck , Krzysztof Warzocha , Luis Araujo , Claudia Moreira , Vadim Doronin , Larisa Mendeleeva , Vladimir Vorobyev , Andrej Vranovsky , Adrian Alegre , Mercedes Gironella , Marta Sonia Gonzalez Perez , Carmen Montes , Enrique Ocio , Paula Rodriguez , Mats Hardling , Birgitta Lauri , Ming-Chung Wang , Su-Peng Yeh , Mutlu Arat , Fatih Demirkan , Zafer Gulbas , Sevgi Kalayoglu Besisik , Ihsan Karadogan , Tulin Tuglular , Ali Unal , Filiz Vural , Jonathan Sive , Matthew Streetly , Kwee Yong , Jason Tache
The Lancet ( IF 98.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/s0140-6736(19)32556-5 Michel Attal , Paul G Richardson , S Vincent Rajkumar , Jesus San-Miguel , Meral Beksac , Ivan Spicka , Xavier Leleu , Fredrik Schjesvold , Philippe Moreau , Meletios A Dimopoulos , Jeffrey Shang-Yi Huang , Jiri Minarik , Michele Cavo , H Miles Prince , Sandrine Macé , Kathryn P Corzo , Frank Campana , Solenn Le-Guennec , Franck Dubin , Kenneth C Anderson , Michel Attal , Paul G. Richardson , Vincent Rajkumar , Jesus San-Miguel , Meral Beksac , Ivan Spicka , Xavier Leleu , Fredrik Schjesvold , Philippe Moreau , Meletios A. Dimopoulos , Jeffrey Shang-Yi Huang , Jiri Minarik , Michele Cavo , H. Miles Prince , Sandrine Macé , Kathryn P. Corzo , Frank Campana , Solenn Le-Guennec , Franck Dubin , Kenneth C. Anderson , Simon Harrison , Wojt Janowski , Ian Kerridge , Andrew Spencer , Michel Delforge , Karel Fostier , Philip Vlummens , Ka Lung Wu , Richard Leblanc , Michel Pavic , Michael Sebag , Roman Hajek , Vladimir Maisnar , Ludek Pour , Henrik Gregersen , Lotfi Benbouker , Denis Caillot , Martine Escoffre-Barbe , Thierry Facon , Laurent Frenzel , Cyrille Hulin , Lionel Karlin , Brigitte Kolb , Brigitte Pegourie , Aurore Perrot , Mourad Tiab , Laure Vincent , Dietger Niederwieser , Achilles Anagnostopoulos , Sosana Delimpasi , Marie-Christine Kyrtsonis , Anargyros Symeonidis , Arpad Illes , Gabor Mikala , Zsolt Nagy , Sara Bringen , Paolo Corradini , Ciceri Fabio , Roberto Lemoli , Anna Liberati , Chiara Nozzoli , Renato Zambello , Shinsuke Iida , Takashi Ikeda , Satoshi Iyama , Morio Matsumoto , Chihiro Shimazaki , Kazutaka Sunami , Kenshi Suzuki , Michihiro Uchiyama , Youngil Koh , Kihyun Kim , Jae Hoon Lee , Chang-Ki Min , Hillary Blacklock , Hugh Goodman , Annette Neylon , David Simpson , Sebastian Grosicki , Artur Jurczyszyn , Adam Walter-Croneck , Krzysztof Warzocha , Luis Araujo , Claudia Moreira , Vadim Doronin , Larisa Mendeleeva , Vladimir Vorobyev , Andrej Vranovsky , Adrian Alegre , Mercedes Gironella , Marta Sonia Gonzalez Perez , Carmen Montes , Enrique Ocio , Paula Rodriguez , Mats Hardling , Birgitta Lauri , Ming-Chung Wang , Su-Peng Yeh , Mutlu Arat , Fatih Demirkan , Zafer Gulbas , Sevgi Kalayoglu Besisik , Ihsan Karadogan , Tulin Tuglular , Ali Unal , Filiz Vural , Jonathan Sive , Matthew Streetly , Kwee Yong , Jason Tache
|
BACKGROUND
Isatuximab is a monoclonal antibody that binds a specific epitope on the human CD38 receptor and has antitumour activity via multiple mechanisms of action. In a previous phase 1b study, around 65% of patients with relapsed and refractory multiple myeloma achieved an overall response with a combination of isatuximab with pomalidomide and low-dose dexamethasone. The aim of this study was to determine the progression-free survival benefit of isatuximab plus pomalidomide and dexamethasone compared with pomalidomide and dexamethasone in patients with relapsed and refractory multiple myeloma.
METHODS
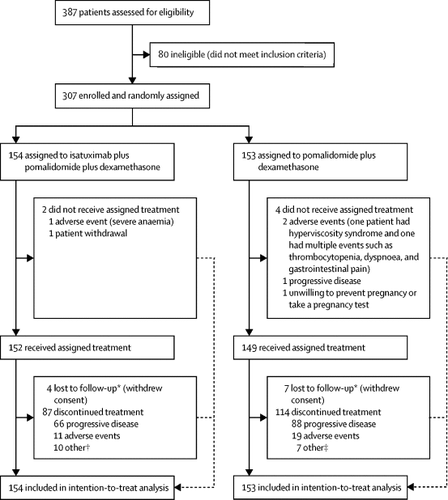
We did a randomised, multicentre, open-label, phase 3 study at 102 hospitals in 24 countries in Europe, North America, and the Asia-Pacific regions. Eligible participants were adult patients with relapsed and refractory multiple myeloma who had received at least two previous lines of treatment, including lenalidomide and a proteasome inhibitor. Patients were excluded if they were refractory to previous treatment with an anti-CD38 monoclonal antibody. We randomly assigned patients (1:1) to either isatuximab 10 mg/kg plus pomalidomide 4 mg plus dexamethasone 40 mg (20 mg for patients aged ≥75 years), or pomalidomide 4 mg plus dexamethasone 40 mg. Randomisation was done using interactive response technology and stratified according to the number of previous lines of treatment (2-3 vs >3) and age (<75 years vs ≥75 years). Treatments were assigned based on a permuted blocked randomisation scheme with a block size of four. The isatuximab-pomalidomide-dexamethasone group received isatuximab intravenously on days 1, 8, 15, and 22 in the first 28-day cycle, then on days 1 and 15 in subsequent cycles. Both groups received oral pomalidomide on days 1 to 21 in each cycle, and oral or intravenous dexamethasone on days 1, 8, 15, and 22 of each cycle. Treatment continued until disease progression, unacceptable toxicity, or consent withdrawal. Dose reductions for adverse reactions were permitted for pomalidomide and dexamethasone, but not for isatuximab. The primary endpoint was progression-free survival, determined by an independent response committee and assessed in the intention-to-treat population. Safety was assessed in all participants who received at least one dose of study drug. This study is registered at ClinicalTrials.gov, number NCT02990338.
FINDINGS
Between Jan 10, 2017, and Feb 2, 2018, we randomly assigned 307 patients to treatment: 154 to isatuximab-pomalidomide-dexamethasone, and 153 to pomalidomide-dexamethasone. At a median follow-up of 11·6 months (IQR 10·1-13·9), median progression-free survival was 11·5 months (95% CI 8·9-13·9) in the isatuximab-pomalidomide-dexamethasone group versus 6·5 months (4·5-8·3) in the pomalidomide-dexamethasone group; hazard ratio 0·596, 95% CI 0·44-0·81; p=0·001 by stratified log-rank test. The most frequent treatment-emergent adverse events (any grade; isatuximab-pomalidomide-dexamethasone vs pomalidomide-dexamethasone) were infusion reactions (56 [38%] vs 0), upper respiratory tract infections (43 [28%] vs 26 [17%]), and diarrhoea (39 [26%] vs 29 [20%]). Adverse events with a fatal outcome were reported in 12 patients (8%) in the isatuximab-pomalidomide-dexamethasone group and 14 (9%) in the pomalidomide-dexamethasone group. Deaths due to treatment-related adverse events were reported for one patient (<1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
INTERPRETATION
The addition of isatuximab to pomalidomide-dexamethasone significantly improves progression-free survival in patients with relapsed and refractory multiple myeloma. Isatuximab is an important new treatment option for the management of relapsed and refractory myeloma, particularly for patients who become refractory to lenalidomide and a proteasome inhibitor.
FUNDING
Sanofi. VIDEO ABSTRACT.
中文翻译:

复发和难治性多发性骨髓瘤(ICARIA-MM)患者中的Isatuximab加pomalidomide和低剂量地塞米松与pomalidomide和低剂量地塞米松的比较:一项随机,多中心,开放标签的3期研究。
背景技术伊沙妥昔单抗是一种单克隆抗体,可与人CD38受体上的特定表位结合,并通过多种作用机制具有抗肿瘤活性。在先前的1b期研究中,约65%的复发性和难治性多发性骨髓瘤患者通过将isatuximab与pomalidomide和小剂量地塞米松联合使用,获得了总体缓解。这项研究的目的是确定在复发和难治性多发性骨髓瘤患者中,伊沙妥昔单抗加波马利度胺和地塞米松与波马利度胺和地塞米松相比无进展生存获益。方法我们在欧洲,北美和亚太地区的24个国家的102家医院进行了一项随机,多中心,开放标签的3期研究。符合条件的参与者是患有复发性和难治性多发性骨髓瘤的成年患者,他们已经接受了至少两个以前的治疗方案,包括来那度胺和蛋白酶体抑制剂。如果患者对先前使用抗CD38单克隆抗体的治疗无效,则将其排除在外。我们将患者(1:1)随机分配给伊沙妥昔单抗10 mg / kg加泊莫利度4 mg加地塞米松40 mg(≥75岁的患者20 mg)或泊马利度4 mg加地塞米松40 mg。使用互动反应技术进行随机分组,并根据既往治疗方案的数目(2-3对> 3)和年龄(<75岁对≥75岁)进行分层。根据置换的分组随机方案分配治疗方案,分组大小为4。在第28天的第1天,第8天,第15天和第22天,在第28天的第1天,第8天,第15天和第22天,然后在随后的第1天和第15天,静脉注射isatuximab-pomalidomide-地塞米松组。两组在每个周期的第1至21天接受口服pomalidomide,在每个周期的第1、8、15和22天接受口服或静脉地塞米松治疗。继续治疗直至疾病进展,不可接受的毒性或同意撤消。泊洛米度和地塞米松的不良反应剂量可减少,但伊沙昔单抗则不允许。主要终点是无进展生存,由独立反应委员会确定并在意向性治疗人群中评估。在接受至少一剂研究药物的所有参与者中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT02990338。结果在2017年1月10日至2018年2月2日之间,我们随机分配了307例患者进行治疗:154例是伊司沙昔单抗-泊马利度胺-地塞米松,153例是泊马利度胺-地塞米松。在中位随访11·6个月(IQR 10·1-13·9)时,isatuximab-pomalidomide--的中位无进展生存期为11·5个月(95%CI 8·9-13·9)。地塞米松组与pomalidomide-地塞米松组的6·5个月(4·5-8·3)相比;危险比0·596,95%CI 0·44-0·81; 通过分层对数秩检验,p = 0·001。出现治疗最频繁的不良事件(任何等级;伊沙妥昔单抗-泊马利度胺-地塞米松vs泊马利度胺-地塞米松)分别是输液反应(56 [38%] vs 0),上呼吸道感染(43 [28%] vs 26 [17%] ])和腹泻(39 [26%]比29 [20%])。Isatuximab-pomalidomide-地塞米松组中有12例患者(8%)发生致命结果的不良事件,pomalidomide-地塞米松组中有14例(9%)。依沙妥昔单抗-pomalidomide-地塞米松组(败血症)中有一名患者(<1%)报告了因治疗相关不良事件而死亡,而pomalidomide-地塞米松组(肺炎和尿路感染)中有两名患者(<1%)死亡。解释在帕马利度胺-地塞米松中添加伊沙昔单抗可显着改善复发和难治性多发性骨髓瘤患者的无进展生存期。Isatuximab是治疗复发和难治性骨髓瘤的重要新治疗方法,特别是对于来那度胺和蛋白酶体抑制剂难以治疗的患者。资助赛诺菲。视频摘要。
更新日期:2019-12-06
中文翻译:

复发和难治性多发性骨髓瘤(ICARIA-MM)患者中的Isatuximab加pomalidomide和低剂量地塞米松与pomalidomide和低剂量地塞米松的比较:一项随机,多中心,开放标签的3期研究。
背景技术伊沙妥昔单抗是一种单克隆抗体,可与人CD38受体上的特定表位结合,并通过多种作用机制具有抗肿瘤活性。在先前的1b期研究中,约65%的复发性和难治性多发性骨髓瘤患者通过将isatuximab与pomalidomide和小剂量地塞米松联合使用,获得了总体缓解。这项研究的目的是确定在复发和难治性多发性骨髓瘤患者中,伊沙妥昔单抗加波马利度胺和地塞米松与波马利度胺和地塞米松相比无进展生存获益。方法我们在欧洲,北美和亚太地区的24个国家的102家医院进行了一项随机,多中心,开放标签的3期研究。符合条件的参与者是患有复发性和难治性多发性骨髓瘤的成年患者,他们已经接受了至少两个以前的治疗方案,包括来那度胺和蛋白酶体抑制剂。如果患者对先前使用抗CD38单克隆抗体的治疗无效,则将其排除在外。我们将患者(1:1)随机分配给伊沙妥昔单抗10 mg / kg加泊莫利度4 mg加地塞米松40 mg(≥75岁的患者20 mg)或泊马利度4 mg加地塞米松40 mg。使用互动反应技术进行随机分组,并根据既往治疗方案的数目(2-3对> 3)和年龄(<75岁对≥75岁)进行分层。根据置换的分组随机方案分配治疗方案,分组大小为4。在第28天的第1天,第8天,第15天和第22天,在第28天的第1天,第8天,第15天和第22天,然后在随后的第1天和第15天,静脉注射isatuximab-pomalidomide-地塞米松组。两组在每个周期的第1至21天接受口服pomalidomide,在每个周期的第1、8、15和22天接受口服或静脉地塞米松治疗。继续治疗直至疾病进展,不可接受的毒性或同意撤消。泊洛米度和地塞米松的不良反应剂量可减少,但伊沙昔单抗则不允许。主要终点是无进展生存,由独立反应委员会确定并在意向性治疗人群中评估。在接受至少一剂研究药物的所有参与者中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT02990338。结果在2017年1月10日至2018年2月2日之间,我们随机分配了307例患者进行治疗:154例是伊司沙昔单抗-泊马利度胺-地塞米松,153例是泊马利度胺-地塞米松。在中位随访11·6个月(IQR 10·1-13·9)时,isatuximab-pomalidomide--的中位无进展生存期为11·5个月(95%CI 8·9-13·9)。地塞米松组与pomalidomide-地塞米松组的6·5个月(4·5-8·3)相比;危险比0·596,95%CI 0·44-0·81; 通过分层对数秩检验,p = 0·001。出现治疗最频繁的不良事件(任何等级;伊沙妥昔单抗-泊马利度胺-地塞米松vs泊马利度胺-地塞米松)分别是输液反应(56 [38%] vs 0),上呼吸道感染(43 [28%] vs 26 [17%] ])和腹泻(39 [26%]比29 [20%])。Isatuximab-pomalidomide-地塞米松组中有12例患者(8%)发生致命结果的不良事件,pomalidomide-地塞米松组中有14例(9%)。依沙妥昔单抗-pomalidomide-地塞米松组(败血症)中有一名患者(<1%)报告了因治疗相关不良事件而死亡,而pomalidomide-地塞米松组(肺炎和尿路感染)中有两名患者(<1%)死亡。解释在帕马利度胺-地塞米松中添加伊沙昔单抗可显着改善复发和难治性多发性骨髓瘤患者的无进展生存期。Isatuximab是治疗复发和难治性骨髓瘤的重要新治疗方法,特别是对于来那度胺和蛋白酶体抑制剂难以治疗的患者。资助赛诺菲。视频摘要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号