当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical investigation of von Braun and von Braun‐like reactions
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-15 , DOI: 10.1002/qua.26088 François Couty 1 , Oleg N. Burov 2 , Mikhail E. Kletskii 2 , Anton V. Lisovin 2 , Karen Wright 1 , Bruno Drouillat 1 , Sergey V. Kurbatov 2
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-15 , DOI: 10.1002/qua.26088 François Couty 1 , Oleg N. Burov 2 , Mikhail E. Kletskii 2 , Anton V. Lisovin 2 , Karen Wright 1 , Bruno Drouillat 1 , Sergey V. Kurbatov 2
Affiliation
|
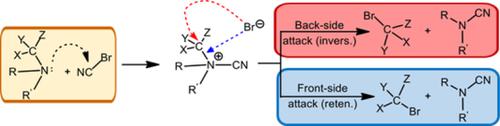
The von Braun reaction, discovered at the dawn of the past century, consists of the reaction between a tertiary amine and cyanogen bromide. It leads to the cleavage of a C─N bond with the formation of an N‐dialkylcyanamide and an alkyl bromide and has been extensively used in organic synthesis. A detailed in silico study (PCM/density functional theory [DFT]/B3LYP/6‐31++G(d,p) calculations) of this venerable reaction has shown that in the first stage a zwitterionic adduct with a multibonded bromine atom is formed. The widely accepted mechanism involving an SN2 reaction occurs in the second step, thus accounting for its selectivity. Quantum chemical calculations were performed for the von Braun‐like reactions in systems formed by cyclic tertiary amines (N‐alkyl azetidines). In these cases, the first stage is almost the same as in the classical von Braun processes, and selective SN2 mechanisms can occur in the second step.
中文翻译:

冯·布劳恩和类似冯·布劳恩反应的理论研究
上个世纪初发现的冯·布劳恩反应由叔胺和溴化氰之间的反应组成。它导致C-N键断裂并形成N-二烷基氰酰胺和烷基溴化物,已广泛用于有机合成中。在计算机上进行的详细研究(PCM /密度泛函理论[DFT] / B3LYP / 6-31 ++ G(d,p)计算)表明,在第一步中,具有多键溴原子的两性离子加合物是形成。在第二步骤中发生了涉及S N 2反应的广为接受的机理,因此考虑了其选择性。在由环叔胺(N-烷基氮杂环丁烷)。在这些情况下,第一阶段与经典的von Braun过程几乎相同,第二阶段可能发生选择性的S N 2机制。
更新日期:2019-12-21
中文翻译:

冯·布劳恩和类似冯·布劳恩反应的理论研究
上个世纪初发现的冯·布劳恩反应由叔胺和溴化氰之间的反应组成。它导致C-N键断裂并形成N-二烷基氰酰胺和烷基溴化物,已广泛用于有机合成中。在计算机上进行的详细研究(PCM /密度泛函理论[DFT] / B3LYP / 6-31 ++ G(d,p)计算)表明,在第一步中,具有多键溴原子的两性离子加合物是形成。在第二步骤中发生了涉及S N 2反应的广为接受的机理,因此考虑了其选择性。在由环叔胺(N-烷基氮杂环丁烷)。在这些情况下,第一阶段与经典的von Braun过程几乎相同,第二阶段可能发生选择性的S N 2机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号