当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Perspectives in the Indole Ring Functionalization using 2‐Indolylmethanols
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-21 , DOI: 10.1002/adsc.201901245 Marino Petrini 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-21 , DOI: 10.1002/adsc.201901245 Marino Petrini 1
Affiliation
|
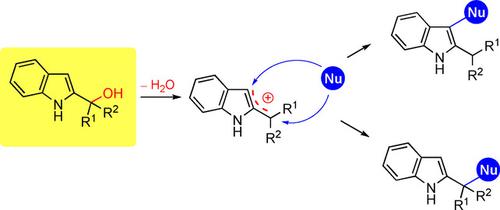
2‐Indolylmethanols have been recently involved in several processes dealing with indole functionalization. These compounds, upon activation by Brønsted or Lewis acids, generate a bidentate electrophilic system amenable to react at the 3‐position or at the benzylic site with a wide range of nucleophilic reagents. The functionalization pattern is affected by the nature of the substituents at the carbinol unit and also depends on the nature of the nucleophile used. Nucleophilic reactants bearing a remote electrophilic site in their structure can be involved in a further ring closure ultimately leading to polycyclic derivatives. This review article summarizes some fundamental aspects of the chemistry of 2‐indolylmethanols with particular attention to those related to asymmetric synthesis.
中文翻译:

使用2-吲哚基甲醇在吲哚环官能化中的新观点
2-吲哚基甲醇最近参与了一些涉及吲哚官能化的过程。这些化合物经布朗斯台德酸或路易斯酸活化后,会生成双齿亲电子体系,该体系可在3位或苄基部位与多种亲核试剂反应。官能化模式受甲醇单元上取代基的性质影响,并且还取决于所用亲核试剂的性质。在其结构中带有较远亲电部位的亲核反应物可参与进一步的闭环反应,最终导致多环衍生物。这篇综述文章总结了2-吲哚基乙醇化学的一些基本方面,特别是那些与不对称合成有关的方面。
更新日期:2020-01-22
中文翻译:

使用2-吲哚基甲醇在吲哚环官能化中的新观点
2-吲哚基甲醇最近参与了一些涉及吲哚官能化的过程。这些化合物经布朗斯台德酸或路易斯酸活化后,会生成双齿亲电子体系,该体系可在3位或苄基部位与多种亲核试剂反应。官能化模式受甲醇单元上取代基的性质影响,并且还取决于所用亲核试剂的性质。在其结构中带有较远亲电部位的亲核反应物可参与进一步的闭环反应,最终导致多环衍生物。这篇综述文章总结了2-吲哚基乙醇化学的一些基本方面,特别是那些与不对称合成有关的方面。







































 京公网安备 11010802027423号
京公网安备 11010802027423号