Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Complete Structure and Model of Tel1ATM from Chaetomium thermophilum Reveals a Robust Autoinhibited ATP State.
Structure ( IF 4.4 ) Pub Date : 2019-11-15 , DOI: 10.1016/j.str.2019.10.013 Marijke Jansma 1 , Christian Linke-Winnebeck 1 , Sebastian Eustermann 1 , Katja Lammens 1 , Dirk Kostrewa 1 , Kristina Stakyte 1 , Claudia Litz 1 , Brigitte Kessler 1 , Karl-Peter Hopfner 1
Structure ( IF 4.4 ) Pub Date : 2019-11-15 , DOI: 10.1016/j.str.2019.10.013 Marijke Jansma 1 , Christian Linke-Winnebeck 1 , Sebastian Eustermann 1 , Katja Lammens 1 , Dirk Kostrewa 1 , Kristina Stakyte 1 , Claudia Litz 1 , Brigitte Kessler 1 , Karl-Peter Hopfner 1
Affiliation
|
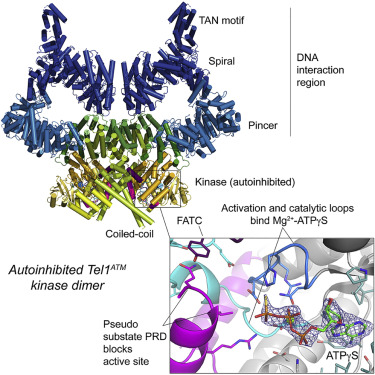
Tel1 (ATM in humans) is a large kinase that resides in the cell in an autoinhibited dimeric state and upon activation orchestrates the cellular response to DNA damage. We report the structure of an endogenous Tel1 dimer from Chaetomium thermophilum. Major parts are at 2.8 Å resolution, including the kinase active site with ATPγS bound, and two different N-terminal solenoid conformations are at 3.4 Å and 3.6 Å, providing a side-chain model for 90% of the Tel1 polypeptide. We show that the N-terminal solenoid has DNA binding activity, but that its movements are not coupled to kinase activation. Although ATPγS and catalytic residues are poised for catalysis, the kinase resides in an autoinhibited state. The PIKK regulatory domain acts as a pseudo-substrate, blocking direct access to the site of catalysis. The structure allows mapping of human cancer mutations and defines mechanisms of autoinhibition at near-atomic resolution.
中文翻译:

嗜热Chaetomium thermophilum的Tel1ATM的近乎完整的结构和模型显示了强大的自动抑制ATP状态。
Tel1(人类中的ATM)是一种大激酶,以自抑制二聚体状态存在于细胞中,一旦激活,就会协调细胞对DNA损伤的反应。我们报告了嗜热毛壳菌的内源性Tel1二聚体的结构。主要部分的分辨力为2.8Å,包括与ATPγS结合的激酶活性位点,两个不同的N末端螺线管构象分别为3.4Å和3.6Å,为90%的Tel1多肽提供了侧链模型。我们显示,N末端螺线管具有DNA结合活性,但其运动不与激酶激活偶联。尽管ATPγS和催化残基已准备好进行催化,但该激酶仍处于自抑制状态。PIKK调节域充当伪底物,阻止直接进入催化位点。
更新日期:2019-11-15
中文翻译:

嗜热Chaetomium thermophilum的Tel1ATM的近乎完整的结构和模型显示了强大的自动抑制ATP状态。
Tel1(人类中的ATM)是一种大激酶,以自抑制二聚体状态存在于细胞中,一旦激活,就会协调细胞对DNA损伤的反应。我们报告了嗜热毛壳菌的内源性Tel1二聚体的结构。主要部分的分辨力为2.8Å,包括与ATPγS结合的激酶活性位点,两个不同的N末端螺线管构象分别为3.4Å和3.6Å,为90%的Tel1多肽提供了侧链模型。我们显示,N末端螺线管具有DNA结合活性,但其运动不与激酶激活偶联。尽管ATPγS和催化残基已准备好进行催化,但该激酶仍处于自抑制状态。PIKK调节域充当伪底物,阻止直接进入催化位点。







































 京公网安备 11010802027423号
京公网安备 11010802027423号