当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrolysis of mixtures of methane and ethane: activation of methane with the aid of radicals generated from ethane
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1039/c9re00400a Hitoshi Ogihara 1, 2, 3, 4 , Hiroki Tajima 1, 2, 3, 4 , Hideki Kurokawa 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1039/c9re00400a Hitoshi Ogihara 1, 2, 3, 4 , Hiroki Tajima 1, 2, 3, 4 , Hideki Kurokawa 1, 2, 3, 4
Affiliation
|
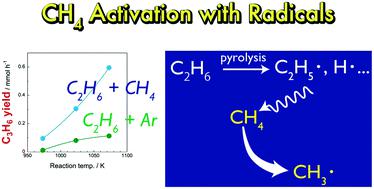
Direct chemical conversion of methane (CH4) has been actively researched in order to use natural gas as a chemical resource. However, the high stability of CH4 molecules hinders the chemical conversion of CH4. In this study, we investigated pyrolysis of mixtures of CH4 and ethane (C2H6) at 973–1073 K. Even though CH4 alone did not react in the temperature range, mixtures of CH4/C2H6 and of Ar/C2H6 showed different pyrolysis behaviours; the co-existence of CH4 significantly increased yields of propylene (C3H6), propane (C3H8) and toluene. Mass spectrometry analysis using 13C-labeled CH4 revealed that carbon contained in CH4 was incorporated into the pyrolysis products. The results suggested that CH4 was activated with the aid of C2H6. We assumed that CH4 was attacked by radical species generated from pyrolysis of C2H6 and was converted into methyl radicals. The CH4-derived methyl radicals were incorporated into pyrolysis products via radical reactions. This study clarified that CH4 can be activated by radicals generated from co-existing molecules without the help of catalysts or extremely high temperature.
中文翻译:

甲烷和乙烷混合物的热解:借助乙烷产生的自由基活化甲烷
为了利用天然气作为化学资源,已经积极研究了甲烷(CH 4)的直接化学转化。然而,CH的高稳定性4个分子阻碍CH的化学转化4。在这项研究中,我们研究了CH 4和乙烷(C 2 H 6)的混合物在973–1073 K时的热解。即使单独的CH 4在该温度范围内不发生反应,CH 4 / C 2 H 6的混合物和Ar / C 2 H 6表现出不同的热解行为。CH 4的共存显着提高了丙烯(C 3 H 6),丙烷(C 3 H 8)和甲苯的收率。使用质谱分析13 C标记的CH 4显示包含在CH碳4被并入热解产物。结果表明,CH 4被C 2 H 6活化。我们假设CH 4受到C 2 H 6热解产生的自由基的攻击,并转化为甲基自由基。CH 4衍生的甲基被掺入热解产物中通过自由基反应。这项研究表明,CH 4可以被共存分子产生的自由基活化,而无需借助催化剂或极高的温度。
更新日期:2019-11-14
中文翻译:

甲烷和乙烷混合物的热解:借助乙烷产生的自由基活化甲烷
为了利用天然气作为化学资源,已经积极研究了甲烷(CH 4)的直接化学转化。然而,CH的高稳定性4个分子阻碍CH的化学转化4。在这项研究中,我们研究了CH 4和乙烷(C 2 H 6)的混合物在973–1073 K时的热解。即使单独的CH 4在该温度范围内不发生反应,CH 4 / C 2 H 6的混合物和Ar / C 2 H 6表现出不同的热解行为。CH 4的共存显着提高了丙烯(C 3 H 6),丙烷(C 3 H 8)和甲苯的收率。使用质谱分析13 C标记的CH 4显示包含在CH碳4被并入热解产物。结果表明,CH 4被C 2 H 6活化。我们假设CH 4受到C 2 H 6热解产生的自由基的攻击,并转化为甲基自由基。CH 4衍生的甲基被掺入热解产物中通过自由基反应。这项研究表明,CH 4可以被共存分子产生的自由基活化,而无需借助催化剂或极高的温度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号