当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of naturally occurring serine/cysteine variations on the structure and function of Pseudomonas metallothioneins.
Metallomics ( IF 2.9 ) Pub Date : 2019-11-15 , DOI: 10.1039/c9mt00213h Jelena Habjanič 1 , Serge Chesnov , Oliver Zerbe , Eva Freisinger
Metallomics ( IF 2.9 ) Pub Date : 2019-11-15 , DOI: 10.1039/c9mt00213h Jelena Habjanič 1 , Serge Chesnov , Oliver Zerbe , Eva Freisinger
Affiliation
|
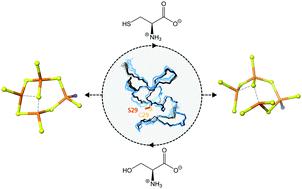
Metallothioneins (MTs), small cysteine-rich metal-binding proteins, support the viability of organisms under normal physiological conditions and help them to respond to different environmental stressors. Upon metal coordination (e.g. ZnII, CdII, CuI) they form characteristic polynuclear metal–thiolate clusters that are known for their high thermodynamic stability and kinetic lability. However, despite numerous studies, it is still not understood how MTs modulate their metal-binding properties. Pseudomonas MTs are an emerging subclass of bacterial MTs, distinct for their high number of His residues and for several unique features such as an intrinsically disordered long C-terminal tail and multiple variations in the number and nature of coordinating amino acids. These variations might provide the bacteria with a functional advantage derived from evolutionary adaptation to heterogeneous environments. Nearly 90% of the known Pseudomonas MT sequences feature a central YC![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) xxC motif, that is altered to YC
xxC motif, that is altered to YC![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif) xxC in the rest. We demonstrate that the additional Cys residue serves as a coordinating ligand without influencing the metal-binding capacity, the overall metal-binding stability or the structure. However, the additional ligand changes intra-cluster dynamics and, as a consequence, modulates metal transfer reactions that could be functionally advantageous in vivo.
xxC in the rest. We demonstrate that the additional Cys residue serves as a coordinating ligand without influencing the metal-binding capacity, the overall metal-binding stability or the structure. However, the additional ligand changes intra-cluster dynamics and, as a consequence, modulates metal transfer reactions that could be functionally advantageous in vivo.
中文翻译:

天然存在的丝氨酸/半胱氨酸变异对假单胞菌金属硫蛋白的结构和功能的影响。
金属硫蛋白(MTs)是富含半胱氨酸的小金属结合蛋白,在正常的生理条件下支持生物的生存能力,并帮助它们应对不同的环境压力。在金属配位(例如Zn II,Cd II,Cu I)作用下,它们会形成特征性的多核金属-硫醇盐簇,这些簇因其高热力学稳定性和动力学稳定性而闻名。然而,尽管进行了大量研究,但仍不了解MT如何调节其金属结合性能。假单胞菌MT是细菌MT的新兴亚类,其高数量的His残基和几个独特的特征,例如内在无序的长C末端尾巴和配位氨基酸的数量和性质的多种变化,使其具有明显的区别。这些变异可能会为细菌提供从进化适应异质环境中获得的功能优势。大约90%的已知假单胞菌MT序列具有中心YC![[C与合并下线]](https://www.rsc.org/images/entities/char_0043_0332.gif) xxC基序,该基序已更改为YC
xxC基序,该基序已更改为YC![[S与合并下线]](https://www.rsc.org/images/entities/char_0053_0332.gif) 其余部分为xxC。我们证明了额外的Cys残基充当配体,而不会影响金属结合能力,整体金属结合稳定性或结构。但是,另外的配体改变了簇内动力学,因此调节了金属转移反应,这在体内可能是功能上有利的。
其余部分为xxC。我们证明了额外的Cys残基充当配体,而不会影响金属结合能力,整体金属结合稳定性或结构。但是,另外的配体改变了簇内动力学,因此调节了金属转移反应,这在体内可能是功能上有利的。
更新日期:2019-11-15
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) xxC motif, that is altered to YC
xxC motif, that is altered to YC![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif) xxC in the rest. We demonstrate that the additional Cys residue serves as a coordinating ligand without influencing the metal-binding capacity, the overall metal-binding stability or the structure. However, the additional ligand changes intra-cluster dynamics and, as a consequence, modulates metal transfer reactions that could be functionally advantageous in vivo.
xxC in the rest. We demonstrate that the additional Cys residue serves as a coordinating ligand without influencing the metal-binding capacity, the overall metal-binding stability or the structure. However, the additional ligand changes intra-cluster dynamics and, as a consequence, modulates metal transfer reactions that could be functionally advantageous in vivo.
中文翻译:

天然存在的丝氨酸/半胱氨酸变异对假单胞菌金属硫蛋白的结构和功能的影响。
金属硫蛋白(MTs)是富含半胱氨酸的小金属结合蛋白,在正常的生理条件下支持生物的生存能力,并帮助它们应对不同的环境压力。在金属配位(例如Zn II,Cd II,Cu I)作用下,它们会形成特征性的多核金属-硫醇盐簇,这些簇因其高热力学稳定性和动力学稳定性而闻名。然而,尽管进行了大量研究,但仍不了解MT如何调节其金属结合性能。假单胞菌MT是细菌MT的新兴亚类,其高数量的His残基和几个独特的特征,例如内在无序的长C末端尾巴和配位氨基酸的数量和性质的多种变化,使其具有明显的区别。这些变异可能会为细菌提供从进化适应异质环境中获得的功能优势。大约90%的已知假单胞菌MT序列具有中心YC
![[C与合并下线]](https://www.rsc.org/images/entities/char_0043_0332.gif) xxC基序,该基序已更改为YC
xxC基序,该基序已更改为YC![[S与合并下线]](https://www.rsc.org/images/entities/char_0053_0332.gif) 其余部分为xxC。我们证明了额外的Cys残基充当配体,而不会影响金属结合能力,整体金属结合稳定性或结构。但是,另外的配体改变了簇内动力学,因此调节了金属转移反应,这在体内可能是功能上有利的。
其余部分为xxC。我们证明了额外的Cys残基充当配体,而不会影响金属结合能力,整体金属结合稳定性或结构。但是,另外的配体改变了簇内动力学,因此调节了金属转移反应,这在体内可能是功能上有利的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号