Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Katanin Grips the β-Tubulin Tail through an Electropositive Double Spiral to Sever Microtubules.
Developmental Cell ( IF 10.7 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.devcel.2019.10.010 Elena A Zehr 1 , Agnieszka Szyk 1 , Ewa Szczesna 1 , Antonina Roll-Mecak 2
Developmental Cell ( IF 10.7 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.devcel.2019.10.010 Elena A Zehr 1 , Agnieszka Szyk 1 , Ewa Szczesna 1 , Antonina Roll-Mecak 2
Affiliation
|
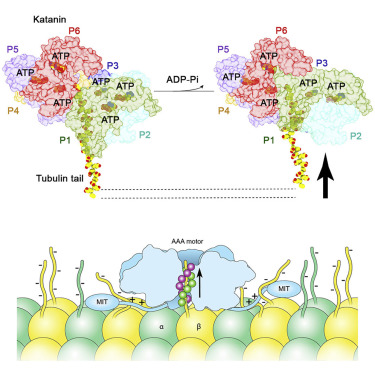
The AAA ATPase katanin severs microtubules. It is critical in cell division, centriole biogenesis, and neuronal morphogenesis. Its mutation causes microcephaly. The microtubule templates katanin hexamerization and activates its ATPase. The structural basis for these activities and how they lead to severing is unknown. Here, we show that β-tubulin tails are necessary and sufficient for severing. Cryoelectron microscopy (cryo-EM) structures reveal the essential tubulin tail glutamates gripped by a double spiral of electropositive loops lining the katanin central pore. Each spiral couples allosterically to the ATPase and binds alternating, successive substrate residues, with consecutive residues coordinated by adjacent protomers. This tightly couples tail binding, hexamerization, and ATPase activation. Hexamer structures in different states suggest an ATPase-driven, ratchet-like translocation of the tubulin tail through the pore. A disordered region outside the AAA core anchors katanin to the microtubule while the AAA motor exerts the forces that extract tubulin dimers and sever the microtubule.
中文翻译:

Katanin 通过带正电的双螺旋抓住 β-微管蛋白尾部以切断微管。
AAA ATP酶剑蛋白切断微管。它对于细胞分裂、中心粒生物发生和神经元形态发生至关重要。它的突变会导致小头畸形。微管模板剑蛋白六聚化并激活其 ATP 酶。这些活动的结构基础以及它们如何导致切断尚不清楚。在这里,我们证明 β-微管蛋白尾部对于切断来说是必要且充分的。冷冻电子显微镜 (cryo-EM) 结构揭示了必需的微管蛋白尾部谷氨酸被卡塔宁中心孔内衬的双螺旋正电环所抓住。每个螺旋以变构方式与 ATP 酶结合,并结合交替的连续底物残基,连续残基由相邻原体协调。这与尾部结合、六聚化和 ATP 酶激活紧密结合。不同状态的六聚体结构表明,微管蛋白尾部通过 ATP 酶驱动、棘轮状易位通过孔。 AAA 核心外部的无序区域将剑蛋白锚定在微管上,而 AAA 马达则施加提取微管蛋白二聚体并切断微管的力。
更新日期:2019-11-14
中文翻译:

Katanin 通过带正电的双螺旋抓住 β-微管蛋白尾部以切断微管。
AAA ATP酶剑蛋白切断微管。它对于细胞分裂、中心粒生物发生和神经元形态发生至关重要。它的突变会导致小头畸形。微管模板剑蛋白六聚化并激活其 ATP 酶。这些活动的结构基础以及它们如何导致切断尚不清楚。在这里,我们证明 β-微管蛋白尾部对于切断来说是必要且充分的。冷冻电子显微镜 (cryo-EM) 结构揭示了必需的微管蛋白尾部谷氨酸被卡塔宁中心孔内衬的双螺旋正电环所抓住。每个螺旋以变构方式与 ATP 酶结合,并结合交替的连续底物残基,连续残基由相邻原体协调。这与尾部结合、六聚化和 ATP 酶激活紧密结合。不同状态的六聚体结构表明,微管蛋白尾部通过 ATP 酶驱动、棘轮状易位通过孔。 AAA 核心外部的无序区域将剑蛋白锚定在微管上,而 AAA 马达则施加提取微管蛋白二聚体并切断微管的力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号