当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Estimating Systematic Error and Uncertainty in Ab Initio Thermochemistry: II. ATOMIC(hc) Enthalpies of Formation for a Large Set of Hydrocarbons.
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jctc.9b00974 Dirk Bakowies 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.jctc.9b00974 Dirk Bakowies 1
Affiliation
|
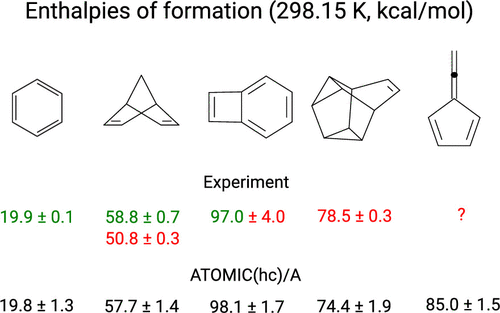
ATOMIC is a thermochemistry protocol geared toward larger molecules with first-row atoms. It implements Pople's concept of bond separation reactions in an ab initio fashion and so enhances the accuracy of midlevel composite models for atomization energies. Recently we have introduced ATOMIC(hc), a model for applications to hydrocarbons, that estimates bias and uncertainty for each of the components contributing to the ATOMIC bottom-of-the-well atomization energy ( Bakowies , D. J. Chem. Theory Comput. 2019 , 15 , 5230 - 5251 ). Here we scrutinize the remaining components of the ATOMIC protocol, including midlevel composite models to approximate the complete-basis set (CBS) limit of CCSD(T) as well as zero-point energies (ZPEs) and thermal enthalpy increments that are evaluated from scaled harmonic MP2 frequencies. Potential errors relating to imperfections in MP2 geometries and ZPEs are estimated using auxiliary information obtained from geometry optimizations and frequency calculations at the density functional (B3LYP) level. Overall corrections to and uncertainties of enthalpies of formation are obtained from summation and error propagation, respectively. The error and uncertainty model is validated with accurate data from the Active Thermochemical Tables (ATcT) and compared to earlier statistical assessments for the G3/99 benchmark. The proposed model is a welcome alternative to statistical assessment, first because it does not depend on comparison with experiment, second because it recognizes the expected scaling of error with system size, and third because it provides a detailed account of the importance of various contributions to overall error and uncertainty. The evaluation of ZPEs from scaled harmonic frequencies expectedly emerges as the leading source of uncertainty if highly accurate composite models are used to treat the electronic problem, but uncertainties are usually balanced with those arising from computationally more attractive B level (B1...B6) models to estimate the CBS limit of CCSD(T). ATOMIC(hc) enthalpies of formation, complete with uncertainty estimates, are reported for 161 hydrocarbons ranging in size from methane (CH4) to [8]circulene (C32H16) and tetra-tert-butyltetrahedrane (C20H36). Experimental data are available for 127 molecules but cannot be reconciled with theory in 37 cases. Theory helps to identify the more accurate among conflicting experimental values in 11 cases and emerges as a valuable complement to experiment also for larger molecules, provided that fair estimates of uncertainty are available.
中文翻译:

从头算热化学中估计系统误差和不确定性:II。大量烃的原子形成焓。
ATOMIC是一种热化学规程,适用于具有第一行原子的较大分子。它从头开始实现了Pople的键分离反应概念,从而提高了中级复合模型雾化能量的准确性。最近,我们引入了ATOMIC(hc),这是一种适用于碳氢化合物的模型,该模型可以估算导致ATOMIC井底雾化能量的每个成分的偏差和不确定性(Bakowies,DJ Chem.Theory Comput.2019, 15、5230-5251)。在这里,我们仔细检查ATOMIC协议的其余组件,包括中层复合模型,以近似估算CCSD(T)的完全基集(CBS)极限以及零点能量(ZPE)和热焓增量(通过缩放比例评估) MP2谐波频率。使用从几何优化和密度函数(B3LYP)级别的频率计算获得的辅助信息,可以估算与MP2几何和ZPE中的缺陷有关的潜在误差。形成焓的整体校正和不确定性分别从求和和误差传播中获得。误差和不确定性模型已使用来自主动热化学表(ATcT)的准确数据进行了验证,并与G3 / 99基准的早期统计评估进行了比较。提议的模型是统计评估的一种受欢迎的替代方法,首先是因为它不依赖于与实验的比较,其次是因为它认识到系统尺寸的预期误差缩放,第三,因为它详细说明了各种因素对总体误差和不确定性的重要性。如果使用高度精确的复合模型来处理电子问题,则根据比例谐波频率对ZPE的评估有望成为不确定性的主要来源,但是不确定性通常与计算上更具吸引力的B级所引起的不确定性保持平衡(B1 ... B6)模型来估计CCSD(T)的CBS限制。报告了161种碳氢化合物的形成焓(不确定性估计值),甲烷的大小从甲烷(CH4)到[8]环戊烯(C32H16)和四叔丁基四面体(C20H36)不等。有127个分子的实验数据,但在37个案例中与理论值不一致。
更新日期:2019-12-21
中文翻译:

从头算热化学中估计系统误差和不确定性:II。大量烃的原子形成焓。
ATOMIC是一种热化学规程,适用于具有第一行原子的较大分子。它从头开始实现了Pople的键分离反应概念,从而提高了中级复合模型雾化能量的准确性。最近,我们引入了ATOMIC(hc),这是一种适用于碳氢化合物的模型,该模型可以估算导致ATOMIC井底雾化能量的每个成分的偏差和不确定性(Bakowies,DJ Chem.Theory Comput.2019, 15、5230-5251)。在这里,我们仔细检查ATOMIC协议的其余组件,包括中层复合模型,以近似估算CCSD(T)的完全基集(CBS)极限以及零点能量(ZPE)和热焓增量(通过缩放比例评估) MP2谐波频率。使用从几何优化和密度函数(B3LYP)级别的频率计算获得的辅助信息,可以估算与MP2几何和ZPE中的缺陷有关的潜在误差。形成焓的整体校正和不确定性分别从求和和误差传播中获得。误差和不确定性模型已使用来自主动热化学表(ATcT)的准确数据进行了验证,并与G3 / 99基准的早期统计评估进行了比较。提议的模型是统计评估的一种受欢迎的替代方法,首先是因为它不依赖于与实验的比较,其次是因为它认识到系统尺寸的预期误差缩放,第三,因为它详细说明了各种因素对总体误差和不确定性的重要性。如果使用高度精确的复合模型来处理电子问题,则根据比例谐波频率对ZPE的评估有望成为不确定性的主要来源,但是不确定性通常与计算上更具吸引力的B级所引起的不确定性保持平衡(B1 ... B6)模型来估计CCSD(T)的CBS限制。报告了161种碳氢化合物的形成焓(不确定性估计值),甲烷的大小从甲烷(CH4)到[8]环戊烯(C32H16)和四叔丁基四面体(C20H36)不等。有127个分子的实验数据,但在37个案例中与理论值不一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号