当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Cu(II) and Zn(II) on the Reductive Dissolution of Pb(IV) Oxide
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-11-14 , DOI: 10.1021/acs.estlett.9b00612 Weiyi Pan 1 , Lia Schattner 1 , Justin Guilak 2 , Daniel Giammar 1
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-11-14 , DOI: 10.1021/acs.estlett.9b00612 Weiyi Pan 1 , Lia Schattner 1 , Justin Guilak 2 , Daniel Giammar 1
Affiliation
|
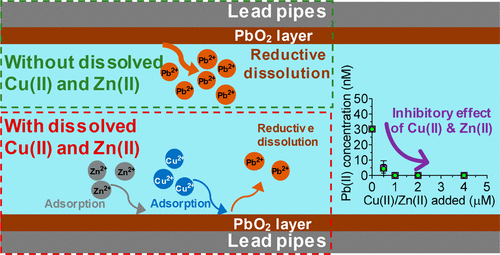
Dissolved lead (Pb) concentrations in drinking water are mainly controlled by Pb-containing minerals in corrosion scales on lead service lines. Lead(IV) oxide (PbO2) solids can control lead concentrations at very low values, but they are stable only in the presence of a free chlorine residual. When free chlorine is depleted, PbO2 undergoes reductive dissolution. Zinc (Zn) and copper (Cu) may accumulate on lead-containing corrosion scales through adsorption or precipitation processes in ways that affect the rate and extent of PbO2 dissolution. The effects of Cu(II) and Zn(II) on the reductive dissolution of PbO2 were explored in batch experiments with PbO2 solids and aqueous Cu(II) and Zn(II). By investigating dissolved Pb(II) concentrations with different Cu(II) and Zn(II) loadings (0–4 μM) and under different pH conditions (5.5–9.5), we found a robust inhibitory effect of Cu(II) and Zn(II) on the reductive dissolution of PbO2. Zn(II) exhibited inhibition that was stronger than that of Cu(II). Characterization of the PbO2 after reactions was consistent with adsorption of Cu(II) and Zn(II), leading to inhibition of PbO2 dissolution. Under all conditions studied, α-PbO2 dissolved faster than β-PbO2.
中文翻译:

Cu(II)和Zn(II)对Pb(IV)氧化物还原溶解的影响
饮用水中溶解的铅(Pb)浓度主要受铅服务管线上腐蚀范围内的含铅矿物质控制。氧化铅(IV)(PbO 2)固体可以将铅浓度控制在非常低的水平,但它们仅在存在游离氯残余物的情况下才稳定。当游离氯耗尽时,PbO 2经历还原溶解。锌(Zn)和铜(Cu)可能通过吸附或沉淀过程以影响PbO 2溶解速率和程度的方式积聚在含铅腐蚀垢中。铜(II)和上的PbO的还原溶解的Zn(II)的效果2在间歇实验进行了探索用的PbO 2固体和含水的Cu(II)和Zn(II)。通过研究不同的Cu(II)和Zn(II)负载量(0–4μM)和在不同的pH条件(5.5–9.5)下溶解的Pb(II)浓度,我们发现了Cu(II)和Zn的强大抑制作用(II)关于PbO 2的还原溶解。Zn(II)表现出比Cu(II)更强的抑制作用。反应后PbO 2的表征与Cu(II)和Zn(II)的吸附一致,从而抑制了PbO 2的溶解。下所研究的所有条件下,α-PBO 2溶解比β-PBO更快2。
更新日期:2019-11-14
中文翻译:

Cu(II)和Zn(II)对Pb(IV)氧化物还原溶解的影响
饮用水中溶解的铅(Pb)浓度主要受铅服务管线上腐蚀范围内的含铅矿物质控制。氧化铅(IV)(PbO 2)固体可以将铅浓度控制在非常低的水平,但它们仅在存在游离氯残余物的情况下才稳定。当游离氯耗尽时,PbO 2经历还原溶解。锌(Zn)和铜(Cu)可能通过吸附或沉淀过程以影响PbO 2溶解速率和程度的方式积聚在含铅腐蚀垢中。铜(II)和上的PbO的还原溶解的Zn(II)的效果2在间歇实验进行了探索用的PbO 2固体和含水的Cu(II)和Zn(II)。通过研究不同的Cu(II)和Zn(II)负载量(0–4μM)和在不同的pH条件(5.5–9.5)下溶解的Pb(II)浓度,我们发现了Cu(II)和Zn的强大抑制作用(II)关于PbO 2的还原溶解。Zn(II)表现出比Cu(II)更强的抑制作用。反应后PbO 2的表征与Cu(II)和Zn(II)的吸附一致,从而抑制了PbO 2的溶解。下所研究的所有条件下,α-PBO 2溶解比β-PBO更快2。









































 京公网安备 11010802027423号
京公网安备 11010802027423号