当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30399-0 Gregory L Krauss 1 , Pavel Klein 2 , Christian Brandt 3 , Sang Kun Lee 4 , Ivan Milanov 5 , Maja Milovanovic 6 , Bernhard J Steinhoff 7 , Marc Kamin 8
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30399-0 Gregory L Krauss 1 , Pavel Klein 2 , Christian Brandt 3 , Sang Kun Lee 4 , Ivan Milanov 5 , Maja Milovanovic 6 , Bernhard J Steinhoff 7 , Marc Kamin 8
Affiliation
|
BACKGROUND
More than a third of patients with epilepsy are treatment resistant, and thus new, more effective therapies to achieve seizure freedom are needed. Cenobamate (YKP3089), an investigational antiepileptic drug, has shown broad-spectrum anticonvulsant activity in preclinical studies and seizure models. We aimed to evaluate the safety, efficacy, and tolerability of adjunctive cenobamate in patients with uncontrolled focal (partial)-onset epilepsy. METHODS
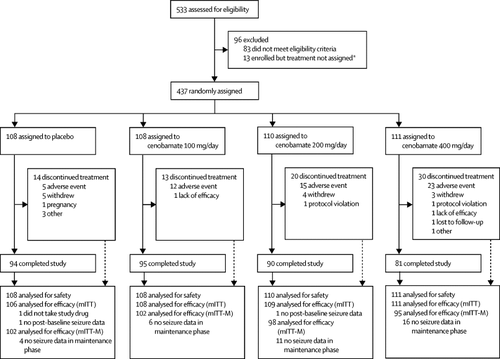
We did a multicentre, double-blind, randomised, placebo-controlled, dose-response study at 107 epilepsy and neurology centres in 16 countries. Adult patients (aged 18-70 years) with focal seizures despite treatment with 1-3 antiepileptic drugs were randomly assigned (1:1:1:1) via an interactive web response system, by block sizes of 4 within each country, to adjuvant once daily oral cenobamate at dose groups of 100 mg, 200 mg, or 400 mg, or placebo following an 8-week baseline assessment. Patients, investigators, and study personnel were masked to treatment assignment. The study included a 6-week titration phase and 12-week maintenance phase. The primary efficacy outcomes were percentage change in 28-day focal seizure frequency (focal aware motor, focal impaired awareness, or focal to bilateral tonic-clonic seizures) from baseline analysed in the modified intention-to-treat population (≥1 dose and any post-baseline seizure data) and responder rates (≥50% reduction) analysed in the maintenance phase population (≥1 dose in the maintenance phase and any maintenance phase seizure data). The primary efficacy outcomes were analysed using a hierarchal step-down procedure comparing 200 mg versus placebo, 400 mg versus placebo, then 100 mg versus placebo. Safety and tolerability were compared descriptively across treatment groups for all randomised patients. This study is registered with ClinicalTrials.gov, number NCT01866111. FINDINGS
Between July 31, 2013, and June 22, 2015, 437 patients were randomly assigned to either placebo (n=108) or cenobamate 100 mg (n=108), 200 mg (n=110), or 400 mg (n=111). Of these patients, 434 (106 [98%] in placebo group, 108 [100%] in 100 mg group, 109 [99%] in 200 mg group, and 111 [100%] in 400 mg group) were included in the modified intention-to-treat population, and 397 (102 [94%] in placebo group, 102 [94%] in 100 mg group, 98 [89%] in 200 mg group, and 95 [86%] in 400 mg group) were included in the modified intention-to-treat maintenance phase population. Median percentage changes in seizure frequency were -24·0% (IQR -45·0 to -7·0%) for the placebo group compared with -35·5% (-62·5 to -15·0%; p=0·0071) for the 100 mg dose group, -55·0% (-73·0 to -23·0%; p<0·0001) for the 200 mg dose group, and -55·0% (-85·0 to -28·0%; p<0·0001) for the 400 mg dose group. Responder rates during the maintenance phase were 25% (26 of 102 patients) for the placebo group compared with 40% (41 of 102; odds ratio 1·97, 95% CI 1·08-3·56; p=0·0365) for the 100 mg dose group, 56% (55 of 98; 3·74, 2·06-6·80; p<0·0001) for the 200 mg dose group, and 64% (61 of 95; 5·24, 2·84-9·67; p<0·0001) for the 400 mg dose group. Treatment-emergent adverse events occurred in 76 (70%) of 108 patients in the placebo group, 70 (65%) of 108 in the 100 mg group, 84 (76%) of 110 in the 200 mg group, and 100 (90%) of 111 in the 400 mg group. Treatment-emergent adverse events led to discontinuation in five (5%) patients in the placebo group, 11 (10%) in the 100 mg dose group, 15 (14%) in the 200 mg dose group, and 22 (20%) in the 400 mg dose group. One serious case of drug reaction with eosinophilia and systemic symptoms occurred in the 200 mg cenobamate group. No deaths were reported. INTERPRETATION
Adjunctive cenobamate reduced focal (partial)-onset seizure frequency, in a dose-related fashion. Treatment-emergent adverse events were most frequent in the highest dose group. Cenobamate appears to be an effective treatment option in patients with uncontrolled focal seizures. FUNDING
SK Life Science.
中文翻译:

非控制性局灶性癫痫患者辅助性 cenobamate (YKP3089) 的安全性和有效性:一项多中心、双盲、随机、安慰剂对照、剂量反应试验
背景 超过三分之一的癫痫患者对治疗具有抗性,因此需要新的、更有效的疗法来实现无癫痫发作。Cenobamate (YKP3089) 是一种研究性抗癫痫药物,已在临床前研究和癫痫发作模型中显示出广谱抗惊厥活性。我们旨在评估非控制性局灶性(部分)发作性癫痫患者辅助使用西诺巴酯的安全性、有效性和耐受性。方法 我们在 16 个国家的 107 个癫痫和神经病学中心进行了一项多中心、双盲、随机、安慰剂对照、剂量反应研究。尽管使用 1-3 种抗癫痫药物治疗但仍出现局灶性癫痫发作的成年患者(18-70 岁)通过交互式网络响应系统随机分配(1:1:1:1),在每个国家/地区按区块大小为 4,在 8 周的基线评估后,以 100 毫克、200 毫克或 400 毫克的剂量组每天一次口服 cenobamate 或安慰剂作为佐剂。患者、研究人员和研究人员对治疗分配不知情。该研究包括 6 周的滴定阶段和 12 周的维持阶段。主要疗效结局是在改良意向治疗人群(≥1 剂量和任何剂量)中分析的 28 天局灶性癫痫发作频率(局灶性运动、局灶性意识障碍或局灶性至双侧强直阵挛发作)与基线相比的百分比变化。基线后癫痫发作数据)和在维持阶段人群(维持阶段≥1 剂量和任何维持阶段癫痫发作数据)中分析的反应率(减少≥50%)。主要疗效结果使用分层递减程序进行分析,比较 200 毫克与安慰剂,400 毫克对比安慰剂,然后 100 毫克对比安慰剂。对所有随机患者的治疗组之间的安全性和耐受性进行了描述性比较。本研究已在 ClinicalTrials.gov 注册,编号 NCT01866111。结果 在 2013 年 7 月 31 日至 2015 年 6 月 22 日期间,437 名患者被随机分配到安慰剂组 (n=108) 或西诺贝特 100 mg (n=108)、200 mg (n=110) 或 400 mg (n= 111)。在这些患者中,434 名(安慰剂组 106 [98%]、100 mg 组 108 [100%]、200 mg 组 109 [99%] 和 400 mg 组 111 [100%])被纳入改良意向治疗人群,397 人(安慰剂组 102 人 [94%],100 毫克组 102 人 [94%],200 毫克组 98 人 [89%],400 毫克组 95 人 [86%] ) 被包括在改良的意向治疗维持阶段人群中。安慰剂组癫痫发作频率变化的中位数百分比为 -24·0%(IQR -45·0 至 -7·0%),而安慰剂组为 -35·5%(-62·5 至 -15·0%;p= 0·0071)对于 100 mg 剂量组,-55·0%(-73·0 至 -23·0%;p<0·0001)对于 200 mg 剂量组,-55·0%(-85 ·0至-28·0%;p<0·0001)对于400mg剂量组。安慰剂组在维持阶段的反应率为 25%(102 名患者中的 26 名),而安慰剂组为 40%(102 名中的 41 名;优势比 1·97,95% CI 1·08-3·56;p=0·0365 ) 对于 100 mg 剂量组,56% (55 of 98; 3·74, 2·06-6·80; p<0·0001) 为 200 mg 剂量组,64% (61 of 95; 5· 24, 2·84-9·67;p<0·0001)对于400mg剂量组。安慰剂组 108 名患者中有 76 名 (70%) 发生治疗紧急不良事件,100 毫克组 108 名患者中有 70 名 (65%) 发生,200 毫克组 110 名患者中有 84 名 (76%),400 mg 组 111 例中的 100 例 (90%)。治疗中出现的不良事件导致安慰剂组 5 名 (5%) 患者、100 毫克剂量组 11 名 (10%) 患者、200 毫克剂量组 15 名 (14%) 和 22 名 (20%) 患者停药在 400 mg 剂量组中。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。200 毫克剂量组中有 15 名 (14%),400 毫克剂量组中有 22 名 (20%)。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。200 毫克剂量组中有 15 名 (14%),400 毫克剂量组中有 22 名 (20%)。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。
更新日期:2020-01-01
中文翻译:

非控制性局灶性癫痫患者辅助性 cenobamate (YKP3089) 的安全性和有效性:一项多中心、双盲、随机、安慰剂对照、剂量反应试验
背景 超过三分之一的癫痫患者对治疗具有抗性,因此需要新的、更有效的疗法来实现无癫痫发作。Cenobamate (YKP3089) 是一种研究性抗癫痫药物,已在临床前研究和癫痫发作模型中显示出广谱抗惊厥活性。我们旨在评估非控制性局灶性(部分)发作性癫痫患者辅助使用西诺巴酯的安全性、有效性和耐受性。方法 我们在 16 个国家的 107 个癫痫和神经病学中心进行了一项多中心、双盲、随机、安慰剂对照、剂量反应研究。尽管使用 1-3 种抗癫痫药物治疗但仍出现局灶性癫痫发作的成年患者(18-70 岁)通过交互式网络响应系统随机分配(1:1:1:1),在每个国家/地区按区块大小为 4,在 8 周的基线评估后,以 100 毫克、200 毫克或 400 毫克的剂量组每天一次口服 cenobamate 或安慰剂作为佐剂。患者、研究人员和研究人员对治疗分配不知情。该研究包括 6 周的滴定阶段和 12 周的维持阶段。主要疗效结局是在改良意向治疗人群(≥1 剂量和任何剂量)中分析的 28 天局灶性癫痫发作频率(局灶性运动、局灶性意识障碍或局灶性至双侧强直阵挛发作)与基线相比的百分比变化。基线后癫痫发作数据)和在维持阶段人群(维持阶段≥1 剂量和任何维持阶段癫痫发作数据)中分析的反应率(减少≥50%)。主要疗效结果使用分层递减程序进行分析,比较 200 毫克与安慰剂,400 毫克对比安慰剂,然后 100 毫克对比安慰剂。对所有随机患者的治疗组之间的安全性和耐受性进行了描述性比较。本研究已在 ClinicalTrials.gov 注册,编号 NCT01866111。结果 在 2013 年 7 月 31 日至 2015 年 6 月 22 日期间,437 名患者被随机分配到安慰剂组 (n=108) 或西诺贝特 100 mg (n=108)、200 mg (n=110) 或 400 mg (n= 111)。在这些患者中,434 名(安慰剂组 106 [98%]、100 mg 组 108 [100%]、200 mg 组 109 [99%] 和 400 mg 组 111 [100%])被纳入改良意向治疗人群,397 人(安慰剂组 102 人 [94%],100 毫克组 102 人 [94%],200 毫克组 98 人 [89%],400 毫克组 95 人 [86%] ) 被包括在改良的意向治疗维持阶段人群中。安慰剂组癫痫发作频率变化的中位数百分比为 -24·0%(IQR -45·0 至 -7·0%),而安慰剂组为 -35·5%(-62·5 至 -15·0%;p= 0·0071)对于 100 mg 剂量组,-55·0%(-73·0 至 -23·0%;p<0·0001)对于 200 mg 剂量组,-55·0%(-85 ·0至-28·0%;p<0·0001)对于400mg剂量组。安慰剂组在维持阶段的反应率为 25%(102 名患者中的 26 名),而安慰剂组为 40%(102 名中的 41 名;优势比 1·97,95% CI 1·08-3·56;p=0·0365 ) 对于 100 mg 剂量组,56% (55 of 98; 3·74, 2·06-6·80; p<0·0001) 为 200 mg 剂量组,64% (61 of 95; 5· 24, 2·84-9·67;p<0·0001)对于400mg剂量组。安慰剂组 108 名患者中有 76 名 (70%) 发生治疗紧急不良事件,100 毫克组 108 名患者中有 70 名 (65%) 发生,200 毫克组 110 名患者中有 84 名 (76%),400 mg 组 111 例中的 100 例 (90%)。治疗中出现的不良事件导致安慰剂组 5 名 (5%) 患者、100 毫克剂量组 11 名 (10%) 患者、200 毫克剂量组 15 名 (14%) 和 22 名 (20%) 患者停药在 400 mg 剂量组中。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。200 毫克剂量组中有 15 名 (14%),400 毫克剂量组中有 22 名 (20%)。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。200 毫克剂量组中有 15 名 (14%),400 毫克剂量组中有 22 名 (20%)。200mg cenobamate组发生1例严重的药物反应伴嗜酸性粒细胞增多和全身症状。没有死亡报告。解释 辅助性cenobamate 以剂量相关的方式降低了局灶性(部分)发作的癫痫发作频率。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。治疗中出现的不良事件在最高剂量组中最为常见。对于无法控制的局灶性癫痫发作的患者,Cenobamate 似乎是一种有效的治疗选择。资助 SK 生命科学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号