当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: pilot phase of a randomised controlled trial.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-14 , DOI: 10.1016/s1470-2045(19)30635-7 Leeya F Pinder 1 , Groesbeck P Parham 2 , Partha Basu 3 , Richard Muwonge 3 , Eric Lucas 3 , Namakau Nyambe 4 , Catherine Sauvaget 3 , Mulindi H Mwanahamuntu 5 , Rengaswamy Sankaranarayanan 6 , Walter Prendiville 3
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-14 , DOI: 10.1016/s1470-2045(19)30635-7 Leeya F Pinder 1 , Groesbeck P Parham 2 , Partha Basu 3 , Richard Muwonge 3 , Eric Lucas 3 , Namakau Nyambe 4 , Catherine Sauvaget 3 , Mulindi H Mwanahamuntu 5 , Rengaswamy Sankaranarayanan 6 , Walter Prendiville 3
Affiliation
|
BACKGROUND
Cryotherapy is standard practice for treating patients with cervical precancer in see-and-treat programmes in low-income and middle-income countries (LMICs). Because of logistical difficulties with cryotherapy (eg, the necessity, costs, and supply chain difficulties of refrigerant gas; equipment failure; and treatment duration >10 min), a battery-operated thermal ablator that is lightweight and portable has been developed. We aimed to compare thermal ablation using the new device with cryotherapy.
METHODS
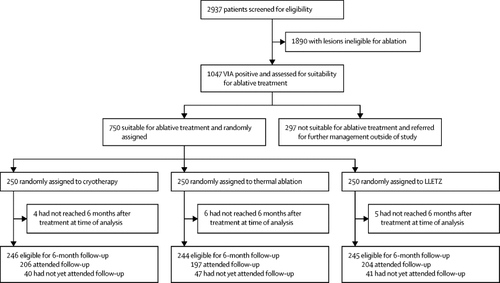
We report the pilot phase of a randomised controlled trial in routine screen-and-treat clinics providing cervical screening using visual inspection with acetic acid (VIA) in Lusaka, Zambia. We recruited non-pregnant women, aged 25 years or older, who were eligible for ablative therapy. We randomly assigned participants (1:1:1) to thermal ablation, cryotherapy, or large loop excision of the transformation zone (LLETZ), using computer-generated allocation. The randomisation was concealed but the nurses providing treatment and the participants were unmasked. Thermal ablation was achieved using the Liger thermal ablator (using 1-5 overlapping applications of the probe heated to 100°C, each application lasting for 40 s), cryotherapy was carried out using the double-freeze technique (freeze for 3 min, thaw for 5 min, and freeze again for 3 min), and LLETZ (using a large loop driven by an electro-surgical unit to excise the transformation zone) was done under local anaesthesia. The primary endpoint was treatment success, defined as either human papillomavirus (HPV) type-specific clearance among participants who were positive for the same HPV type at baseline, or a negative VIA test at 6-month follow-up, if the baseline HPV test was negative. Per protocol analyses were done. Enrolment for the full trial is ongoing. Here, we present findings from a prespecified pilot phase of the full trial. The final analysis of the full trial will assess non-inferiority of the groups for the primary efficacy endpoint. The study is registered with ClinicalTrials.gov, number NCT02956239.
FINDINGS
Between Aug 2, 2017, and Jan 15, 2019, 750 participants were randomly assigned (250 per group). 206 (84%) participants in the cryotherapy group, 197 (81%) in the thermal ablation group, and 204 (84%) in the LLETZ group attended the 6-month follow-up examination. Treatment success was reported in 120 (60%) of 200 participants in the cryotherapy group, 123 (64%) of 192 in the thermal ablation group, and 134 (67%) of 199 in the LLETZ group (p=0·31). Few participants complained of moderate to severe pain in any group immediately after the procedure (six [2%] of 250 in the cryotherapy group, four [2%] of 250 in the thermal ablation group, and five [2%] of 250 in the LLETZ group) and 2 weeks after the procedure (one [<1%] of 241 in the cryotherapy group, none of 242 in the thermal ablation group, and two [<1%] of 237 in the LLETZ group). None of the participants reported any complication requiring medical consultation or admission to hospital.
INTERPRETATION
Results from this pilot study preliminarily suggest that thermal ablation has similar treatment success to cryotherapy, without the practical disadvantages of providing cryotherapy in an LMIC. However, the study was not powered to establish the similarity between the techniques, and results from the ongoing randomised controlled trial are need to confirm these results.
FUNDING
US National Institutes of Health.
中文翻译:

热消融与冷冻疗法或环切除术治疗经醋酸测试目视检查呈宫颈癌前期阳性的女性:随机对照试验的试点阶段。
背景冷冻疗法是低收入和中等收入国家(LMIC)的“即诊即治”项目中治疗宫颈癌前病变患者的标准做法。由于冷冻治疗的后勤困难(例如,制冷剂气体的必要性、成本和供应链困难;设备故障;以及治疗持续时间>10分钟),开发了一种重量轻、便携式的电池供电热消融器。我们的目的是比较使用新设备的热消融与冷冻疗法。方法 我们报告了在赞比亚卢萨卡的常规筛查和治疗诊所中进行的一项随机对照试验的试点阶段,这些诊所使用醋酸目视检查(VIA)提供子宫颈筛查。我们招募了年龄 25 岁或以上、有资格接受消融治疗的非孕妇。我们使用计算机生成的分配,将参与者随机分配(1:1:1)接受热消融、冷冻疗法或变性区大环切除术 (LLETZ)。随机分组是隐藏的,但提供治疗的护士和参与者都是公开的。使用Liger热消融仪实现热消融(使用加热至100°C的探头重叠应用1-5次,每次应用持续40秒),使用双冷冻技术进行冷冻治疗(冷冻3分钟,解冻) 5分钟,再次冷冻3分钟),LLETZ(使用由电外科装置驱动的大环切除转化区)在局部麻醉下完成。主要终点是治疗成功,定义为基线时相同 HPV 类型呈阳性的参与者中人乳头瘤病毒 (HPV) 类型特异性清除,或者如果基线 HPV 检测结果为阴性,则在 6 个月随访时 VIA 检测结果为阴性呈阴性。 根据协议进行分析。完整试验的注册正在进行中。在这里,我们展示了完整试验的预先指定的试点阶段的结果。完整试验的最终分析将评估各组主要疗效终点的非劣效性。该研究已在 ClinicalTrials.gov 注册,编号为 NCT02956239。结果 2017年8月2日至2019年1月15日期间,750名参与者被随机分配(每组250人)。冷冻治疗组有 206 名参与者(84%),热消融组有 197 名参与者(81%),LLETZ 组有 204 名参与者(84%)参加了 6 个月的随访检查。冷冻治疗组 200 名参与者中有 120 名 (60%) 治疗成功,热消融组 192 名参与者中有 123 名 (64%) 治疗成功,LLETZ 组 199 名参与者中有 134 名 (67%) 治疗成功 (p=0·31) 。在任何组中,几乎没有参与者在手术后立即抱怨中度至重度疼痛(冷冻治疗组 250 人中有 6 人 [2%],热消融组 250 人中有 4 人 [2%],热消融组 250 人中有 5 人 [2%]。 LLETZ 组)和术后 2 周(冷冻治疗组 241 例中有 1 例 [<1%],热消融组 242 例中无例,LLETZ 组 237 例中有 2 例 [<1%])。没有参与者报告任何需要就医或入院的并发症。解释 这项试点研究的结果初步表明热消融与冷冻疗法具有相似的治疗效果,并且没有在中低收入国家中提供冷冻疗法的实际缺点。然而,该研究并不能确定这些技术之间的相似性,需要正在进行的随机对照试验的结果来证实这些结果。资助美国国立卫生研究院。
更新日期:2020-01-04
中文翻译:

热消融与冷冻疗法或环切除术治疗经醋酸测试目视检查呈宫颈癌前期阳性的女性:随机对照试验的试点阶段。
背景冷冻疗法是低收入和中等收入国家(LMIC)的“即诊即治”项目中治疗宫颈癌前病变患者的标准做法。由于冷冻治疗的后勤困难(例如,制冷剂气体的必要性、成本和供应链困难;设备故障;以及治疗持续时间>10分钟),开发了一种重量轻、便携式的电池供电热消融器。我们的目的是比较使用新设备的热消融与冷冻疗法。方法 我们报告了在赞比亚卢萨卡的常规筛查和治疗诊所中进行的一项随机对照试验的试点阶段,这些诊所使用醋酸目视检查(VIA)提供子宫颈筛查。我们招募了年龄 25 岁或以上、有资格接受消融治疗的非孕妇。我们使用计算机生成的分配,将参与者随机分配(1:1:1)接受热消融、冷冻疗法或变性区大环切除术 (LLETZ)。随机分组是隐藏的,但提供治疗的护士和参与者都是公开的。使用Liger热消融仪实现热消融(使用加热至100°C的探头重叠应用1-5次,每次应用持续40秒),使用双冷冻技术进行冷冻治疗(冷冻3分钟,解冻) 5分钟,再次冷冻3分钟),LLETZ(使用由电外科装置驱动的大环切除转化区)在局部麻醉下完成。主要终点是治疗成功,定义为基线时相同 HPV 类型呈阳性的参与者中人乳头瘤病毒 (HPV) 类型特异性清除,或者如果基线 HPV 检测结果为阴性,则在 6 个月随访时 VIA 检测结果为阴性呈阴性。 根据协议进行分析。完整试验的注册正在进行中。在这里,我们展示了完整试验的预先指定的试点阶段的结果。完整试验的最终分析将评估各组主要疗效终点的非劣效性。该研究已在 ClinicalTrials.gov 注册,编号为 NCT02956239。结果 2017年8月2日至2019年1月15日期间,750名参与者被随机分配(每组250人)。冷冻治疗组有 206 名参与者(84%),热消融组有 197 名参与者(81%),LLETZ 组有 204 名参与者(84%)参加了 6 个月的随访检查。冷冻治疗组 200 名参与者中有 120 名 (60%) 治疗成功,热消融组 192 名参与者中有 123 名 (64%) 治疗成功,LLETZ 组 199 名参与者中有 134 名 (67%) 治疗成功 (p=0·31) 。在任何组中,几乎没有参与者在手术后立即抱怨中度至重度疼痛(冷冻治疗组 250 人中有 6 人 [2%],热消融组 250 人中有 4 人 [2%],热消融组 250 人中有 5 人 [2%]。 LLETZ 组)和术后 2 周(冷冻治疗组 241 例中有 1 例 [<1%],热消融组 242 例中无例,LLETZ 组 237 例中有 2 例 [<1%])。没有参与者报告任何需要就医或入院的并发症。解释 这项试点研究的结果初步表明热消融与冷冻疗法具有相似的治疗效果,并且没有在中低收入国家中提供冷冻疗法的实际缺点。然而,该研究并不能确定这些技术之间的相似性,需要正在进行的随机对照试验的结果来证实这些结果。资助美国国立卫生研究院。









































 京公网安备 11010802027423号
京公网安备 11010802027423号