当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative ultrafast spectroscopy and structural analysis of OCP1 and OCP2 from Tolypothrix.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbabio.2019.148120 Valentyna Kuznetsova 1 , Maria Agustina Dominguez-Martin 2 , Han Bao 2 , Sayan Gupta 3 , Markus Sutter 4 , Miroslav Kloz 5 , Mateusz Rebarz 5 , Martin Přeček 5 , Yan Chen 6 , Christopher J Petzold 6 , Corie Y Ralston 3 , Cheryl A Kerfeld 7 , Tomáš Polívka 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.bbabio.2019.148120 Valentyna Kuznetsova 1 , Maria Agustina Dominguez-Martin 2 , Han Bao 2 , Sayan Gupta 3 , Markus Sutter 4 , Miroslav Kloz 5 , Mateusz Rebarz 5 , Martin Přeček 5 , Yan Chen 6 , Christopher J Petzold 6 , Corie Y Ralston 3 , Cheryl A Kerfeld 7 , Tomáš Polívka 1
Affiliation
|
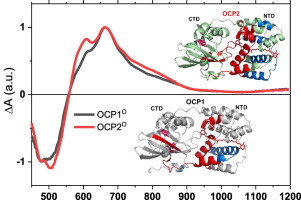
The orange carotenoid protein (OCP) is a structurally and functionally modular photoactive protein involved in cyanobacterial photoprotection. Recently, based on bioinformatic analysis and phylogenetic relationships, new families of OCP have been described, OCP2 and OCPx. The first characterization of the OCP2 showed both faster photoconversion and back-conversion, and lower fluorescence quenching of phycobilisomes relative to the well-characterized OCP1. Moreover, OCP2 is not regulated by the fluorescence recovery protein (FRP). In this work, we present a comprehensive study combining ultrafast spectroscopy and structural analysis to compare the photoactivation mechanisms of OCP1 and OCP2 from Tolypothrix PCC 7601. We show that despite significant differences in their functional characteristics, the spectroscopic properties of OCP1 and OCP2 are comparable. This indicates that the OCP functionality is not directly related to the spectroscopic properties of the bound carotenoid. In addition, the structural analysis by X-ray footprinting reveals that, overall, OCP1 and OCP2 have grossly the same photoactivation mechanism. However, the OCP2 is less reactive to radiolytic labeling, suggesting that the protein is less flexible than OCP1. This observation could explain fast photoconversion of OCP2.
中文翻译:

Tolypothrix 的 OCP1 和 OCP2 的比较超快光谱和结构分析。
橙色类胡萝卜素蛋白(OCP)是一种结构和功能模块化的光活性蛋白,参与蓝藻的光保护。最近,基于生物信息学分析和系统发育关系,描述了OCP的新家族OCP2和OCPx。与充分表征的 OCP1 相比,OCP2 的首次表征显示出更快的光转换和反向转换,以及更低的藻胆体荧光猝灭。此外,OCP2 不受荧光恢复蛋白 (FRP) 的调节。在这项工作中,我们提出了一项结合超快光谱和结构分析的综合研究,以比较 Tolypothrix PCC 7601 中 OCP1 和 OCP2 的光活化机制。我们表明,尽管它们的功能特征存在显着差异,但 OCP1 和 OCP2 的光谱特性具有可比性。这表明 OCP 功能与结合的类胡萝卜素的光谱特性没有直接关系。此外,X射线足迹的结构分析表明,总体而言,OCP1和OCP2具有大致相同的光活化机制。然而,OCP2 对放射分解标记的反应性较低,表明该蛋白质的灵活性不如 OCP1。这一观察结果可以解释 OCP2 的快速光转换。
更新日期:2019-11-14
中文翻译:

Tolypothrix 的 OCP1 和 OCP2 的比较超快光谱和结构分析。
橙色类胡萝卜素蛋白(OCP)是一种结构和功能模块化的光活性蛋白,参与蓝藻的光保护。最近,基于生物信息学分析和系统发育关系,描述了OCP的新家族OCP2和OCPx。与充分表征的 OCP1 相比,OCP2 的首次表征显示出更快的光转换和反向转换,以及更低的藻胆体荧光猝灭。此外,OCP2 不受荧光恢复蛋白 (FRP) 的调节。在这项工作中,我们提出了一项结合超快光谱和结构分析的综合研究,以比较 Tolypothrix PCC 7601 中 OCP1 和 OCP2 的光活化机制。我们表明,尽管它们的功能特征存在显着差异,但 OCP1 和 OCP2 的光谱特性具有可比性。这表明 OCP 功能与结合的类胡萝卜素的光谱特性没有直接关系。此外,X射线足迹的结构分析表明,总体而言,OCP1和OCP2具有大致相同的光活化机制。然而,OCP2 对放射分解标记的反应性较低,表明该蛋白质的灵活性不如 OCP1。这一观察结果可以解释 OCP2 的快速光转换。









































 京公网安备 11010802027423号
京公网安备 11010802027423号