Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy.
Cell ( IF 45.5 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.cell.2019.10.029 Shiping Jiao 1 , Sumit K Subudhi 2 , Ana Aparicio 2 , Zhongqi Ge 3 , Baoxiang Guan 2 , Yuji Miura 2 , Padmanee Sharma 4
Cell ( IF 45.5 ) Pub Date : 2019-11-14 , DOI: 10.1016/j.cell.2019.10.029 Shiping Jiao 1 , Sumit K Subudhi 2 , Ana Aparicio 2 , Zhongqi Ge 3 , Baoxiang Guan 2 , Yuji Miura 2 , Padmanee Sharma 4
Affiliation
|
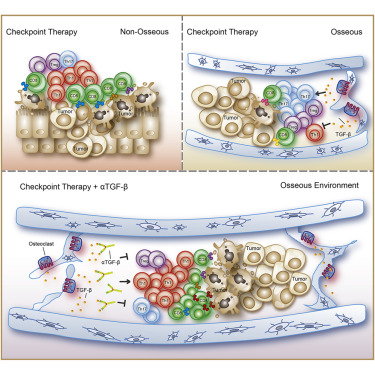
Immune checkpoint therapy (ICT) shows encouraging results in a subset of patients with metastatic castration-resistant prostate cancer (mCRPC) but still elicits a sub-optimal response among those with bone metastases. Analysis of patients' bone marrow samples revealed increased Th17 instead of Th1 subsets after ICT. To further evaluate the different tumor microenvironments, we injected mice with prostate tumor cells either subcutaneously or intraosseously. ICT in the subcutaneous CRPC model significantly increases intra-tumoral Th1 subsets and improves survival. However, ICT fails to elicit an anti-tumor response in the bone CRPC model despite an increase in the intra-tumoral CD4 T cells, which are polarized to Th17 rather than Th1 lineage. Mechanistically, tumors in the bone promote osteoclast-mediated bone resorption that releases TGF-β, which restrains Th1 lineage development. Blocking TGF-β along with ICT increases Th1 subsets and promotes clonal expansion of CD8 T cells and subsequent regression of bone CRPC and improves survival.
中文翻译:

肿瘤微环境听写T辅助谱系极化和对免疫检查点治疗反应的差异。
免疫检查点治疗(ICT)在部分转移性去势抵抗性前列腺癌(mCRPC)患者中显示出令人鼓舞的结果,但在骨转移患者中仍引起次优反应。对患者骨髓样本的分析显示,ICT后,Th17的增加而不是Th1的增加。为了进一步评估不同的肿瘤微环境,我们向小鼠皮下或骨内注射了前列腺肿瘤细胞。皮下CRPC模型中的ICT显着增加了肿瘤内Th1子集并提高了生存率。然而,尽管肿瘤内的CD4 T细胞极化了Th17而不是Th1谱系,但ICT未能在骨CRPC模型中引发抗肿瘤反应。从机制上讲,骨骼中的肿瘤会促进破骨细胞介导的骨吸收,从而释放TGF-β,这限制了Th1血统的发展。与ICT一起阻断TGF-β会增加Th1亚型并促进CD8 T细胞的克隆扩增,并随后使骨CRPC消退并提高生存率。
更新日期:2019-11-14
中文翻译:

肿瘤微环境听写T辅助谱系极化和对免疫检查点治疗反应的差异。
免疫检查点治疗(ICT)在部分转移性去势抵抗性前列腺癌(mCRPC)患者中显示出令人鼓舞的结果,但在骨转移患者中仍引起次优反应。对患者骨髓样本的分析显示,ICT后,Th17的增加而不是Th1的增加。为了进一步评估不同的肿瘤微环境,我们向小鼠皮下或骨内注射了前列腺肿瘤细胞。皮下CRPC模型中的ICT显着增加了肿瘤内Th1子集并提高了生存率。然而,尽管肿瘤内的CD4 T细胞极化了Th17而不是Th1谱系,但ICT未能在骨CRPC模型中引发抗肿瘤反应。从机制上讲,骨骼中的肿瘤会促进破骨细胞介导的骨吸收,从而释放TGF-β,这限制了Th1血统的发展。与ICT一起阻断TGF-β会增加Th1亚型并促进CD8 T细胞的克隆扩增,并随后使骨CRPC消退并提高生存率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号