当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tracking Hidden Binding Pockets Along the Molecular Recognition Path of l-Trp to Indoleamine 2,3-Dioxygenase 1.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-14 , DOI: 10.1002/cmdc.201900529 Francesco A Greco 1 , Elisa Albini 2 , Alice Coletti 1 , Daniela Dolciami 1 , Andrea Carotti 1 , Ciriana Orabona 2 , Ursula Grohmann 2 , Antonio Macchiarulo 1
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-14 , DOI: 10.1002/cmdc.201900529 Francesco A Greco 1 , Elisa Albini 2 , Alice Coletti 1 , Daniela Dolciami 1 , Andrea Carotti 1 , Ciriana Orabona 2 , Ursula Grohmann 2 , Antonio Macchiarulo 1
Affiliation
|
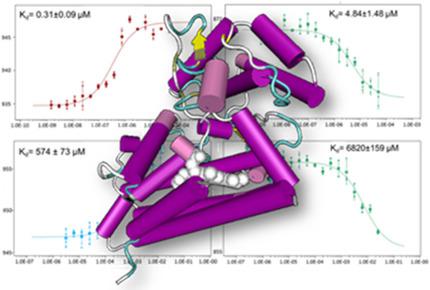
Indoleamine 2,3-dioxygenase 1 (IDO1) catalyzes the oxidative cleavage of l-Tryptophan (l-Trp) to yield N-formyl-kynurenine in the first and rate limiting step of the kynurenine pathway. Bioactive metabolites, involved in the regulation of important immunological responses and neurological processes, are then produced by downstream enzymes along the pathway. Inhibitors of IDO1 are being designed and developed as therapeutic agents for immuno-oncology. In this work, we investigated the molecular recognition path of l-Trp to IDO1, integrating biophysical methods with supervised molecular dynamics (suMD) and mutagenesis experiments. Results allowed disclosing for the first time high and low dissociation constants of l-Trp to IDO1, and the presence of a metastable interaction site located at the upper part of a channel whose borders are defined by the EF-loop and the C-terminal part of the JK-loop. Collectively, our results provide new clues for the design of next-generation IDO1 ligands.
中文翻译:

沿着l-Trp到吲哚胺2,3-二加氧酶1的分子识别路径追踪隐藏的结合口袋。
吲哚胺2,3-二加氧酶1(IDO1)催化1-色氨酸(1-Trp)的氧化裂解,在犬尿氨酸途径的第一步和限速步骤中产生N-甲酰基-犬尿氨酸。然后,通过该途径的下游酶产生参与重要免疫反应和神经过程调节的生物活性代谢物。IDO1抑制剂正在设计和开发中,作为免疫肿瘤学的治疗剂。在这项工作中,我们研究了l-Trp到IDO1的分子识别途径,将生物物理方法与监督分子动力学(suMD)和诱变实验相结合。结果首次公开了I-Trp与IDO1的高解离常数和低解离常数,在通道的上部由EF环和JK环的C端部分定义边界的通道的上部,存在亚稳相互作用位点。总的来说,我们的结果为下一代IDO1配体的设计提供了新的线索。
更新日期:2019-11-14
中文翻译:

沿着l-Trp到吲哚胺2,3-二加氧酶1的分子识别路径追踪隐藏的结合口袋。
吲哚胺2,3-二加氧酶1(IDO1)催化1-色氨酸(1-Trp)的氧化裂解,在犬尿氨酸途径的第一步和限速步骤中产生N-甲酰基-犬尿氨酸。然后,通过该途径的下游酶产生参与重要免疫反应和神经过程调节的生物活性代谢物。IDO1抑制剂正在设计和开发中,作为免疫肿瘤学的治疗剂。在这项工作中,我们研究了l-Trp到IDO1的分子识别途径,将生物物理方法与监督分子动力学(suMD)和诱变实验相结合。结果首次公开了I-Trp与IDO1的高解离常数和低解离常数,在通道的上部由EF环和JK环的C端部分定义边界的通道的上部,存在亚稳相互作用位点。总的来说,我们的结果为下一代IDO1配体的设计提供了新的线索。









































 京公网安备 11010802027423号
京公网安备 11010802027423号