当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Mechanism of Cholesterol Modification of Hedgehog Ligand
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1002/jcc.26097 Nilesh K Banavali 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2019-11-14 , DOI: 10.1002/jcc.26097 Nilesh K Banavali 1
Affiliation
|
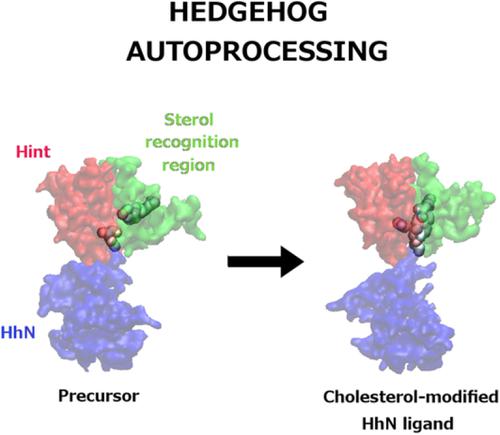
Hedgehog (Hh) proteins are important components of signal transduction pathways involved in animal development, and their defects are implicated in carcinogenesis. Their N‐terminal domain (HhN) acts as a signaling ligand, and their C‐terminal domain (HhC) performs an autocatalytic function of cleaving itself away, while adding a cholesterol moiety to HhN. HhC has two sub‐domains: a hedgehog/intein (hint) domain that primarily performs the autocatalytic activity, and a sterol‐recognition region (SRR) that binds to cholesterol and properly positions it with respect to HhN. The three‐dimensional details of this autocatalytic mechanism remain unknown, as does the structure of the precursor Hh protein. In this study, a complete cholesterol‐bound precursor form of the drosophila Hh precursor is modeled using known crystal structures of HhN and the hint domain, and a hypothesized similarity of SRR to an unrelated but similar‐sized cholesterol binding protein. The restrained geometries and topology switching (RGATS) strategy is then used to predict atomic‐detail pathways for the full autocatalytic reaction starting from the precursor and ending in a cholesterol‐linked HhN domain and a cleaved HhC domain. The RGATS explicit solvent simulations indicate the roles of individual HhC residues in facilitating the reaction, which can be confirmed through mutational experiments. These simulations also provide plausible structural models for the N/S acyl transfer intermediate and the product states of this reaction. This study thus provides a good framework for future computational and experimental studies to develop a full structural and dynamic understanding of Hh autoprocessing. © 2019 Wiley Periodicals, Inc.
中文翻译:

刺猬配体的胆固醇修饰机制
Hedgehog (Hh) 蛋白是参与动物发育的信号转导通路的重要组成部分,它们的缺陷与致癌作用有关。它们的 N 端结构域 (HhN) 作为信号配体,它们的 C 端结构域 (HhC) 执行自我切割的自催化功能,同时向 HhN 添加胆固醇部分。HhC 有两个子结构域:主要执行自催化活性的刺猬蛋白/内含肽(提示)结构域,以及与胆固醇结合并将其相对于 HhN 正确定位的甾醇识别区域(SRR)。这种自催化机制的三维细节仍然未知,前体 Hh 蛋白的结构也是如此。在这项研究中,果蝇 Hh 前体的完整胆固醇结合前体形式使用已知的 HhN 晶体结构和提示域建模,假设 SRR 与不相关但大小相似的胆固醇结合蛋白相似。然后使用受限几何和拓扑转换 (RGATS) 策略来预测完全自催化反应的原子细节途径,从前体开始,以胆固醇连接的 HhN 结构域和裂解的 HhC 结构域结束。RGATS 显式溶剂模拟表明单个 HhC 残基在促进反应中的作用,这可以通过突变实验来证实。这些模拟还为 N/S 酰基转移中间体和该反应的产物状态提供了合理的结构模型。因此,这项研究为未来的计算和实验研究提供了一个良好的框架,以发展对 Hh 自动处理的完整结构和动态理解。© 2019 威利期刊公司。
更新日期:2019-11-14
中文翻译:

刺猬配体的胆固醇修饰机制
Hedgehog (Hh) 蛋白是参与动物发育的信号转导通路的重要组成部分,它们的缺陷与致癌作用有关。它们的 N 端结构域 (HhN) 作为信号配体,它们的 C 端结构域 (HhC) 执行自我切割的自催化功能,同时向 HhN 添加胆固醇部分。HhC 有两个子结构域:主要执行自催化活性的刺猬蛋白/内含肽(提示)结构域,以及与胆固醇结合并将其相对于 HhN 正确定位的甾醇识别区域(SRR)。这种自催化机制的三维细节仍然未知,前体 Hh 蛋白的结构也是如此。在这项研究中,果蝇 Hh 前体的完整胆固醇结合前体形式使用已知的 HhN 晶体结构和提示域建模,假设 SRR 与不相关但大小相似的胆固醇结合蛋白相似。然后使用受限几何和拓扑转换 (RGATS) 策略来预测完全自催化反应的原子细节途径,从前体开始,以胆固醇连接的 HhN 结构域和裂解的 HhC 结构域结束。RGATS 显式溶剂模拟表明单个 HhC 残基在促进反应中的作用,这可以通过突变实验来证实。这些模拟还为 N/S 酰基转移中间体和该反应的产物状态提供了合理的结构模型。因此,这项研究为未来的计算和实验研究提供了一个良好的框架,以发展对 Hh 自动处理的完整结构和动态理解。© 2019 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号