当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure‐activity relationship in the case of intramolecular ortho‐cyclization of aromatic nitroso oxides: Inverted steric effect of substituent in the 2‐R‐C6H4NOO transformation
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-14 , DOI: 10.1002/qua.26094 Alfia R. Yusupova 1 , Ekaterina M. Chainikova 1 , Rustam L. Safiullin 1 , Sergey L. Khursan 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2019-11-14 , DOI: 10.1002/qua.26094 Alfia R. Yusupova 1 , Ekaterina M. Chainikova 1 , Rustam L. Safiullin 1 , Sergey L. Khursan 1
Affiliation
|
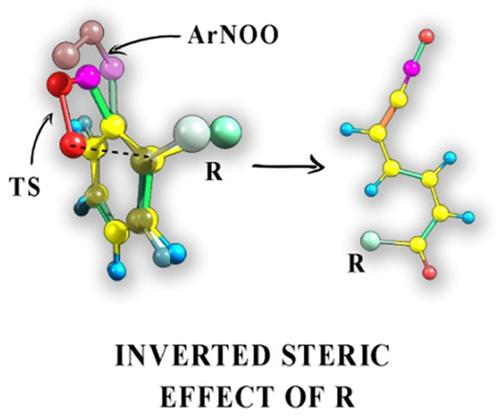
A systematic theoretical study at the M06L/6‐311+G(d, p) level of theory was carried out to calculate the activation barriers ΔH≠ for the intramolecular ortho‐cyclization of aromatic nitroso oxides 2‐R‐C6H4NOO and to reveal the effect of substituent nature and position in the benzene ring on the nitroso oxides reactivity. A set of 24 substituents with widely differing spatial and electronic properties (inductive, resonant, steric effects of R) was studied. The para‐substituent was shown to have little effect on the ΔH≠ value. The full set of effects of the R substituent contributes to the reactivity of ArNOO for 3‐substituted aromatic nitroso oxides. In the case of 5‐substituted ArNOO the Hammett‐type relationship was obtain to describe inductive and resonant effects of R on the ortho‐cyclization reactivity. The ortho‐cyclization for 2‐substituted nitroso oxides is a nontrivial example of the existence of an “inverted” steric effect, when an increase in substituent size accelerates intramolecular transformation. The substituent in position 6 also exhibits an “inverted” steric effect, but it is noticeably weaker than that for 2‐R‐C6H4NOO.
中文翻译:

芳香亚硝基氧化物分子内邻位环化时的结构活性关系:2-R-C6H4NOO转化中取代基的反空间效应
在M06L / 6-311 + G(d,p)的理论水平上进行了系统的理论研究,以计算芳族亚硝基氧化物2-R-C 6 H 4的分子内邻环化的活化能垒ΔH ≠并揭示了取代基的性质和苯环中的位置对亚硝基氧化物反应性的影响。研究了一组24种具有广泛不同的空间和电子特性(R的感应,共振,空间效应)的取代基。的对位-取代显示出具有对Δ的影响不大ħ ≠价值。R取代基的全部作用有助于ArNOO对3取代的芳族亚硝基氧化物的反应性。在5取代的ArNOO的情况下,获得了Hammett型关系来描述R对邻环化反应活性的诱导和共振作用。当取代基尺寸的增加加速分子内转化时,2-取代的亚硝基氧化物的邻位环化是存在“倒置”空间效应的一个重要例子。第6位的取代基也表现出“倒置”空间效应,但明显弱于2- RC 6 H 4 NOO。
更新日期:2020-01-07
中文翻译:

芳香亚硝基氧化物分子内邻位环化时的结构活性关系:2-R-C6H4NOO转化中取代基的反空间效应
在M06L / 6-311 + G(d,p)的理论水平上进行了系统的理论研究,以计算芳族亚硝基氧化物2-R-C 6 H 4的分子内邻环化的活化能垒ΔH ≠并揭示了取代基的性质和苯环中的位置对亚硝基氧化物反应性的影响。研究了一组24种具有广泛不同的空间和电子特性(R的感应,共振,空间效应)的取代基。的对位-取代显示出具有对Δ的影响不大ħ ≠价值。R取代基的全部作用有助于ArNOO对3取代的芳族亚硝基氧化物的反应性。在5取代的ArNOO的情况下,获得了Hammett型关系来描述R对邻环化反应活性的诱导和共振作用。当取代基尺寸的增加加速分子内转化时,2-取代的亚硝基氧化物的邻位环化是存在“倒置”空间效应的一个重要例子。第6位的取代基也表现出“倒置”空间效应,但明显弱于2- RC 6 H 4 NOO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号