Chemical Physics ( IF 2.0 ) Pub Date : 2019-11-13 , DOI: 10.1016/j.chemphys.2019.110603 Haruki Sogawa , Riku Sato , Katsumi Suzuki , Shogo Tomioka , Tomoki Shinzato , Pavel Karpov , Sergey Shulga , Yaroslav Blume , Noriyuki Kurita
|
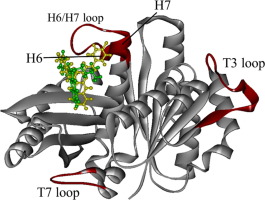
Filamentous temperature-sensitive Z (FtsZ) protein contributes to bacteria cell division, and its inhibition prevents Mycobacteria reproduction. In the present study, Zantrin Z3 and ZZ3 were adopted as inhibitors against FtsZ and their binding properties to FtsZ were investigated, using ab initio molecular simulations based on protein-ligand docking, classical molecular mechanics and ab initio fragment molecular orbital (FMO) calculations. From the total energies of several structures evaluated by the ab initio FMO calculations, we specified the most preferable binding-sites of Z3/ZZ3 to FtsZ and highlighted the key amino acid residues contributing to the binding of these inhibitors at an electronic level. In addition, we revealed the reason why ZZ3 is more potent against FtsZ than Z3 and that ZZ3 is effective for inhibiting the FtsZ aggregation.
中文翻译:

Zantrin抑制剂与细菌细胞分裂蛋白FtsZ的结合位点:分子对接和从头算分子轨道计算
丝状温度敏感Z(FtsZ)蛋白有助于细菌细胞分裂,其抑制作用可防止分枝杆菌繁殖。在本研究中,Zantrin Z3和ZZ3被用作FtsZ的抑制剂,并使用基于蛋白质-配体对接的从头算分子模拟,经典分子力学和从头算片段分子轨道(FMO)计算,研究了它们与FtsZ的结合特性。从头算出的几种结构的总能量FMO计算中,我们指定了Z3 / ZZ3与FtsZ的最优选结合位点,并突出了在电子水平上有助于这些抑制剂结合的关键氨基酸残基。此外,我们揭示了ZZ3对FtsZ的效力比Z3更强的原因,并且ZZ3可以有效抑制FtsZ聚集。







































 京公网安备 11010802027423号
京公网安备 11010802027423号