当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A diastereoselective approach to amino alcohols and application for divergent synthesis of dolastatin 10
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-11-13 , DOI: 10.1039/c9qo01292c Xiao-Di Nie 1, 2, 3, 4 , Zhuo-Ya Mao 1, 2, 3, 4 , Wen Zhou 1, 2, 3, 4 , Chang-Mei Si 1, 2, 3, 4 , Bang-Guo Wei 1, 2, 3, 4 , Guo-Qiang Lin 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-11-13 , DOI: 10.1039/c9qo01292c Xiao-Di Nie 1, 2, 3, 4 , Zhuo-Ya Mao 1, 2, 3, 4 , Wen Zhou 1, 2, 3, 4 , Chang-Mei Si 1, 2, 3, 4 , Bang-Guo Wei 1, 2, 3, 4 , Guo-Qiang Lin 4, 5, 6, 7
Affiliation
|
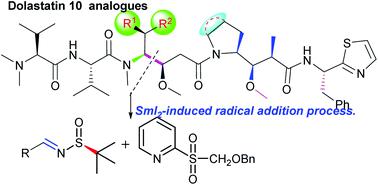
A diastereoselective approach to obtain amino alcohols 10 through SmI2-induced radical addition of chiral imine 8 with 2-(benzyloxymethylsulfonyl)pyridine 9 is described. This approach was easily used for the synthesis of non-natural amino acid 15, a flexible key fragment whose utility was demonstrated in the divergent synthesis of dolastatin 10 (1) and its nine analogues 31a, 31c, 31d, 31e, 31f, 31g, 40a, 40b and 40c were obtained.
中文翻译:

氨基醇的非对映选择性方法及其在dolastatin 10的发散合成中的应用
描述了通过SmI 2诱导的手性亚胺8与2-(苄氧基甲基磺酰基)吡啶9的自由基加成反应获得氨基醇10的非对映选择性方法。这种方法很容易用于合成非天然氨基酸15,这是一种柔性关键片段,其在dolastatin 10(1)及其9个类似物31a,31c,31d,31e,31f,31g,获得40a,40b和40c。
更新日期:2019-11-13
中文翻译:

氨基醇的非对映选择性方法及其在dolastatin 10的发散合成中的应用
描述了通过SmI 2诱导的手性亚胺8与2-(苄氧基甲基磺酰基)吡啶9的自由基加成反应获得氨基醇10的非对映选择性方法。这种方法很容易用于合成非天然氨基酸15,这是一种柔性关键片段,其在dolastatin 10(1)及其9个类似物31a,31c,31d,31e,31f,31g,获得40a,40b和40c。











































 京公网安备 11010802027423号
京公网安备 11010802027423号