当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
US Food and Drug Administration Recommendations on the Use of Surrogate Measures as End Points in New Anti-infective Drug Approvals
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2020-01-01 , DOI: 10.1001/jamainternmed.2019.5451 Spencer Phillips Hey 1, 2 , Aaron S Kesselheim 1, 2 , Pranav Patel 3 , Preeti Mehrotra 4 , John H Powers 5
JAMA Internal Medicine ( IF 22.5 ) Pub Date : 2020-01-01 , DOI: 10.1001/jamainternmed.2019.5451 Spencer Phillips Hey 1, 2 , Aaron S Kesselheim 1, 2 , Pranav Patel 3 , Preeti Mehrotra 4 , John H Powers 5
Affiliation
|
Importance
Regulatory and scientific guidelines stipulate that indirect, surrogate measures of patient benefit, such as a change in microbial culture status, should be used as primary end points only in pivotal trials of chronic conditions that are serious or life threatening and when the experimental therapy is expected to offer substantial benefit compared with available therapy. However, many recent US Food and Drug Administration (FDA) anti-infective drug approvals for acute and/or non-life-threatening diseases have been based on pivotal trials using surrogate measures as primary end points rather than clinical outcomes, such as symptom resolution or survival. Objectives
To review FDA recommendations for primary end points in pivotal trials of new anti-infective drugs and assess the concordance of those recommendations with the regulatory and scientific conditions for the appropriate use of surrogate measures as primary trial outcomes. Evidence Review
All guidance documents for antimicrobial drug development hosted on the FDA website were searched in November 2017; the search was updated in June 2018. For each document, 2 reviewers independently extracted data on the recommended primary end points for a pivotal or phase 3 trial. Findings
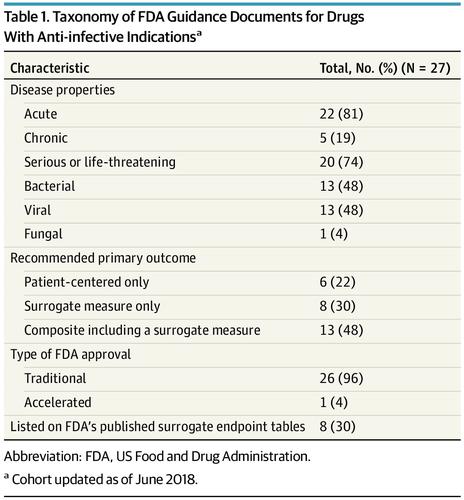
Twenty-two FDA guidance documents met the inclusion criteria, which included recommendations for primary end points in pivotal clinical trials in 27 infectious disease indications. Twenty-one of 27 indications recommended surrogate outcomes as either the sole primary end point or as components of composite end points. None of the recommendations for the use of surrogate measures matched the regulatory and scientific conditions favoring indirect outcomes in place of clinical outcomes. Conclusions and Relevance
The FDA guidance documents for developing new anti-infective agents frequently recommend indirect measures of patient benefit, rather than direct measures of patient benefit, as sole primary end points or components of primary end points. Existing guidance documents should be updated and revised to recommend appropriate clinical outcomes consistent with general scientific and regulatory parameters.
中文翻译:

美国食品和药物管理局关于在新的抗感染药物批准中使用替代措施作为终点的建议
重要性 监管和科学指南规定,仅在严重或危及生命的慢性病的关键试验中,以及当实验治疗与现有疗法相比,有望提供实质性益处。然而,美国食品和药物管理局 (FDA) 最近批准的许多针对急性和/或非危及生命疾病的抗感染药物都是基于关键试验,使用替代措施作为主要终点而不是临床结果,例如症状缓解或生存。目的 审查 FDA 对新抗感染药物关键试验中主要终点的建议,并评估这些建议与适当使用替代措施作为主要试验结果的监管和科学条件的一致性。证据审查 2017 年 11 月检索了 FDA 网站上托管的所有抗菌药物开发指导文件;检索于 2018 年 6 月更新。对于每个文件,2 位评价者独立提取关键或 3 期试验推荐主要终点的数据。结果 22 份 FDA 指导文件符合纳入标准,其中包括对 27 种传染病适应症关键临床试验中主要终点的建议。27 个适应症中有 21 个推荐替代结局作为唯一的主要终点或复合终点的组成部分。没有一项关于使用替代措施的建议符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。使用替代措施的建议均不符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。没有一项关于使用替代措施的建议符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。
更新日期:2020-01-01
中文翻译:

美国食品和药物管理局关于在新的抗感染药物批准中使用替代措施作为终点的建议
重要性 监管和科学指南规定,仅在严重或危及生命的慢性病的关键试验中,以及当实验治疗与现有疗法相比,有望提供实质性益处。然而,美国食品和药物管理局 (FDA) 最近批准的许多针对急性和/或非危及生命疾病的抗感染药物都是基于关键试验,使用替代措施作为主要终点而不是临床结果,例如症状缓解或生存。目的 审查 FDA 对新抗感染药物关键试验中主要终点的建议,并评估这些建议与适当使用替代措施作为主要试验结果的监管和科学条件的一致性。证据审查 2017 年 11 月检索了 FDA 网站上托管的所有抗菌药物开发指导文件;检索于 2018 年 6 月更新。对于每个文件,2 位评价者独立提取关键或 3 期试验推荐主要终点的数据。结果 22 份 FDA 指导文件符合纳入标准,其中包括对 27 种传染病适应症关键临床试验中主要终点的建议。27 个适应症中有 21 个推荐替代结局作为唯一的主要终点或复合终点的组成部分。没有一项关于使用替代措施的建议符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。使用替代措施的建议均不符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。没有一项关于使用替代措施的建议符合有利于间接结果而不是临床结果的监管和科学条件。结论和相关性 FDA 开发新型抗感染药物的指导文件经常推荐间接测量患者获益,而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。而不是直接测量患者获益,作为唯一的主要终点或主要终点的组成部分。应更新和修订现有的指导文件,以推荐与一般科学和监管参数一致的适当临床结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号