当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tableting of hot-melt coated paracetamol granules: Material tableting properties and quality characteristics of the obtained tablets.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.ejps.2019.105121 Ana Milanovic 1 , Ivana Aleksic 1 , Svetlana Ibric 1 , Jelena Parojcic 1 , Sandra Cvijic 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.ejps.2019.105121 Ana Milanovic 1 , Ivana Aleksic 1 , Svetlana Ibric 1 , Jelena Parojcic 1 , Sandra Cvijic 1
Affiliation
|
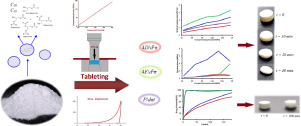
Hot-melt coating (HMC) has been recognized as a promising technique in the production of solid dosage forms e.g., HMC of granules can be applied prior to compression in order to obtain modified drug release or taste masking. However, tableting properties of HMC granules have not been studied yet. In this work, we explored the influence of the lipid coating on granules tableting properties, and assessed quality attributes of the obtained tablets. Paracetamol granules, previously coated with the lipid excipient Precirol® ATO 5 using a hot-melt coating technique in modified fluidized-bed system, were evaluated in terms of work of compression, elastic recovery, tablets tensile strength, detachment stress and ejection stress. Regarding the product quality, tablets content uniformity, friability, disintegration time and drug release properties were tested. Our results demonstrated that tablets made of coated granules exhibited more pronounced elastic behaviour, and increased tensile strength in comparison to tablets made of uncoated granules, suggesting that lipid coating promotes elastic deformation and forms lipid matrix within the tablets. Additionally, low detachment and ejection stresses for tablets made of HMC granules indicated no need to add lubricant prior to tableting process. Evaluation of tablets properties revealed that tablets friability was not influenced by the presence of lipid coating on the compressed granules. However, formation of lipid matrix within the tablets made of HMC granules resulted in prolonged tablet disintegration time, and sustained drug release. Moreover, the performance of lipid matrix tablets, in terms of drug dissolution rate, was relatively insensitive to compression pressure variations in 104-173 MPa range. The obtained results indicate that tableting of HMC granules is a promising technique to obtain sustained release lipid matrix tablets of suitable pharmaceutical-technical properties.
中文翻译:

热熔包衣的扑热息痛颗粒的压片:获得的片剂的材料压片性能和质量特征。
热熔涂层(HMC)已被公认为是固体剂型生产中的一种有前途的技术,例如,可以在压片之前使用颗粒的HMC以获得改善的药物释放或掩味效果。然而,尚未研究HMC颗粒的压片性质。在这项工作中,我们探索了脂质包衣对颗粒压片性能的影响,并评估了所得片剂的质量属性。在压缩,弹性回复,片剂抗张强度,剥离应力和喷射应力方面评估了扑热息痛颗粒,该对乙酰氨基酚颗粒先前已使用脂溶性赋形剂Precirol®ATO 5在改进的流化床系统中采用热熔包衣技术进行包衣。关于产品质量,片剂含量均匀,易碎,测试了崩解时间和药物释放特性。我们的结果表明,与未包衣颗粒制成的片剂相比,由包衣颗粒制成的片剂表现出更明显的弹性行为,并提高了拉伸强度,表明脂质包衣促进了弹性变形并在片剂内形成脂质基质。另外,由HMC颗粒制成的片剂的低剥离应力和喷射应力表明在压片过程之前无需添加润滑剂。片剂性质的评价表明,片剂的脆性不受压缩颗粒上脂质包衣的存在的影响。然而,在由HMC颗粒制成的片剂中脂质基质的形成导致延长的片剂崩解时间和持续的药物释放。而且,脂质基质片的性能,就药物溶解速度而言,它对104-173 MPa范围内的压缩压力变化相对不敏感。获得的结果表明,将HMC颗粒压片是获得具有合适药物技术性能的缓释脂质基质片剂的有前途的技术。
更新日期:2019-11-12
中文翻译:

热熔包衣的扑热息痛颗粒的压片:获得的片剂的材料压片性能和质量特征。
热熔涂层(HMC)已被公认为是固体剂型生产中的一种有前途的技术,例如,可以在压片之前使用颗粒的HMC以获得改善的药物释放或掩味效果。然而,尚未研究HMC颗粒的压片性质。在这项工作中,我们探索了脂质包衣对颗粒压片性能的影响,并评估了所得片剂的质量属性。在压缩,弹性回复,片剂抗张强度,剥离应力和喷射应力方面评估了扑热息痛颗粒,该对乙酰氨基酚颗粒先前已使用脂溶性赋形剂Precirol®ATO 5在改进的流化床系统中采用热熔包衣技术进行包衣。关于产品质量,片剂含量均匀,易碎,测试了崩解时间和药物释放特性。我们的结果表明,与未包衣颗粒制成的片剂相比,由包衣颗粒制成的片剂表现出更明显的弹性行为,并提高了拉伸强度,表明脂质包衣促进了弹性变形并在片剂内形成脂质基质。另外,由HMC颗粒制成的片剂的低剥离应力和喷射应力表明在压片过程之前无需添加润滑剂。片剂性质的评价表明,片剂的脆性不受压缩颗粒上脂质包衣的存在的影响。然而,在由HMC颗粒制成的片剂中脂质基质的形成导致延长的片剂崩解时间和持续的药物释放。而且,脂质基质片的性能,就药物溶解速度而言,它对104-173 MPa范围内的压缩压力变化相对不敏感。获得的结果表明,将HMC颗粒压片是获得具有合适药物技术性能的缓释脂质基质片剂的有前途的技术。









































 京公网安备 11010802027423号
京公网安备 11010802027423号