The Lancet HIV ( IF 16.1 ) Pub Date : 2019-11-11 , DOI: 10.1016/s2352-3018(19)30266-8 Alison D Grant 1 , Salome Charalambous 2 , Mpho Tlali 3 , Aaron S Karat 4 , Susan E Dorman 5 , Christopher J Hoffmann 5 , Suzanne Johnson 6 , Anna Vassall 7 , Gavin J Churchyard 8 , Katherine L Fielding 9
|
Background
Tuberculosis, which is often undiagnosed, is the major cause of death among HIV-positive people. We aimed to test whether the use of a clinical algorithm enabling the initiation of empirical tuberculosis treatment by nurses in primary health-care clinics would reduce mortality compared with standard of care for adults with advanced HIV disease.
Methods
In this open-label cluster-randomised controlled trial, we recruited individuals from 24 primary health-care clinics in South Africa. The clinics were randomly assigned (1:1) to either deliver an intervention or routine care (control) using computer-generated random numbers. Eligible participants were HIV-positive adults (aged ≥18 years) with CD4 counts of 150 cells per μL or less, who had not had antiretroviral therapy (ART) in the past 6 months or tuberculosis treatment in the past 3 months, and did not require urgent hospital referral. In intervention clinics, study nurses assessed participants on the basis of tuberculosis symptoms, body-mass index, point-of-care haemoglobin concentrations, and urine lipoarabinomannan assay results. Participants classified by a study algorithm as having high probability of tuberculosis (positive urine lipoarabinomannan assay, body-mass index <18·5 kg/m2, or haemoglobin concentration <100 g/L) were recommended to start tuberculosis treatment immediately followed by ART 2 weeks later; participants classified as medium probability (tuberculosis symptoms, no high probability criteria) were recommended to have symptom-guided investigation; and participants classified as low probability (no tuberculosis symptoms or high probability criteria) were recommended to start ART immediately. In standard-of-care clinics, participants received treatment in accordance with South African guidelines. Investigators and participants were aware of treatment allocation. The primary outcome was all-cause mortality at 6 months, assessed in the intention-to-treat population. Safety was also analysed in the intention-to treat population. This trial is registered with the ISRCTN registry, ISRCTN35344604, and the South African National Clinical Trials Register, DOH-27-0812-3902.
Findings
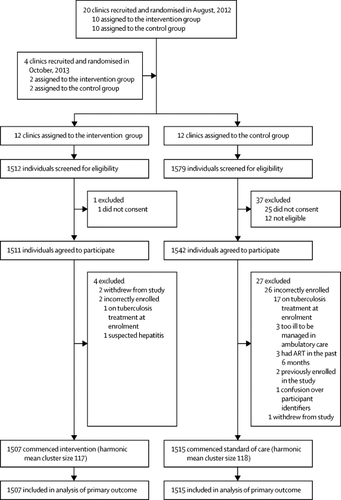
Between Dec 19, 2012, and Dec 18, 2014, 3091 individuals were screened for eligibility, of whom 3053 were recruited, and 3022 (1507 participants in the intervention group and 1515 participants in the control group) were analysed for the primary outcome. 930 (61·7%) of 1507 participants in the intervention group versus 172 (11·4%) of 1515 participants in the control group had started tuberculosis treatment by 2 months. At 6 months, the mortality rate was 19·0 deaths per 100 person-years for the intervention group versus 21·6 deaths per 100 person-years in the control group (unadjusted hazard ratio [HR] 0·92, 95% CI 0·67–1·26, p=0·58; adjusted HR 0·87, 0·61–1·24, p=0·41). 28 (1·9%) of 1507 participants in the intervention group and ten (0·7%) of 1515 participants in the control group reported serious or severe adverse events. Grade 3 or 4 nausea and vomiting was the most common adverse event (ten participants in the intervention group and four participants in the control group). Among participants with adverse events, eight participants (six participants in the intervention group and two participants in the control group) died; none of the six deaths in the intervention group were attributed to the study intervention.
Interpretation
Our intervention substantially increased coverage of tuberculosis treatment in this high-risk population, but did not reduce mortality.
Funding
Joint Global Health Trials (Medical Research Council, Department for International Development, Wellcome Trust).
中文翻译:

针对晚期 HIV 患者的算法指导的经验性结核病治疗(结核病快速通道):一项开放标签、整群随机试验。
背景
结核病常常未被确诊,是艾滋病毒阳性人群的主要死因。我们的目的是测试与患有晚期 HIV 疾病的成年人的护理标准相比,使用临床算法是否能够让初级保健诊所的护士启动经验性结核病治疗会降低死亡率。
方法
在这项开放标签的整群随机对照试验中,我们从南非的 24 家初级保健诊所招募了个人。使用计算机生成的随机数,将诊所随机分配 (1:1) 以提供干预或常规护理(对照)。符合条件的参与者是 HIV 阳性成人(年龄≥18 岁),CD4 细胞计数为 150 个细胞/μL 或更少,在过去 6 个月内未接受过抗逆转录病毒治疗 (ART) 或在过去 3 个月内未接受过结核病治疗,并且没有需要紧急转诊医院。在干预诊所,研究护士根据结核病症状、体重指数、床旁血红蛋白浓度和尿脂阿拉伯甘露聚糖测定结果对参与者进行评估。2个, 或血红蛋白浓度 <100 g/L) 被建议立即开始结核病治疗,然后在 2 周后进行抗逆转录病毒治疗;建议归类为中概率(结核症状,无高概率标准)的参与者进行症状引导调查;建议归类为低概率(无结核症状或高概率标准)的参与者立即开始 ART。在标准护理诊所,参与者根据南非指南接受治疗。调查人员和参与者都知道治疗分配。主要结局是 6 个月时的全因死亡率,在意向治疗人群中进行评估。还在意向治疗人群中分析了安全性。该试验已在 ISRCTN 注册中心注册,ISRCTN35344604,
发现
2012 年 12 月 19 日至 2014 年 12 月 18 日期间,对 3091 人进行了资格筛选,其中 3053 人被招募,并对 3022 人(干预组 1507 名参与者和对照组 1515 名参与者)进行了主要结局分析。干预组 1507 名参与者中的 930 名 (61·7%) 与对照组 1515 名参与者中的 172 名 (11·4%) 在 2 个月时开始了结核病治疗。6 个月时,干预组的死亡率为每 100 人年 19·0 例死亡,而对照组为每 100 人年 21·6 例死亡(未调整风险比 [HR] 0·92,95% CI 0 ·67–1·26,p=0·58;调整后的 HR 0·87,0·61–1·24,p=0·41)。干预组 1507 名参与者中的 28 名 (1·9%) 和对照组 1515 名参与者中的 10 名 (0·7%) 报告了严重或严重的不良事件。3 级或 4 级恶心和呕吐是最常见的不良事件(干预组有 10 名参与者,对照组有 4 名参与者)。在发生不良事件的参与者中,8 名参与者(干预组 6 名参与者和对照组 2 名参与者)死亡;干预组的六例死亡均不归因于研究干预。
解释
我们的干预大大增加了这一高危人群的结核病治疗覆盖率,但并未降低死亡率。
资金
联合全球健康试验(医学研究委员会、国际发展部、Wellcome Trust)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号