Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-11-11 , DOI: 10.1016/s0140-6736(19)32517-6 Stephen A Harrison 1 , Mustafa R Bashir 2 , Cynthia D Guy 2 , Rong Zhou 3 , Cynthia A Moylan 2 , Juan P Frias 4 , Naim Alkhouri 5 , Meena B Bansal 6 , Seth Baum 7 , Brent A Neuschwander-Tetri 8 , Rebecca Taub 9 , Sam E Moussa 10
The Lancet ( IF 98.4 ) Pub Date : 2019-11-11 , DOI: 10.1016/s0140-6736(19)32517-6 Stephen A Harrison 1 , Mustafa R Bashir 2 , Cynthia D Guy 2 , Rong Zhou 3 , Cynthia A Moylan 2 , Juan P Frias 4 , Naim Alkhouri 5 , Meena B Bansal 6 , Seth Baum 7 , Brent A Neuschwander-Tetri 8 , Rebecca Taub 9 , Sam E Moussa 10
Affiliation
|
BACKGROUND
Non-alcoholic steatohepatitis (NASH) is characterised by hepatic steatosis, inflammation, hepatocellular injury, and progressive liver fibrosis. Resmetirom (MGL-3196) is a liver-directed, orally active, selective thyroid hormone receptor-β agonist designed to improve NASH by increasing hepatic fat metabolism and reducing lipotoxicity. We aimed to assess the safety and efficacy of resmetirom in patients with NASH.
METHODS
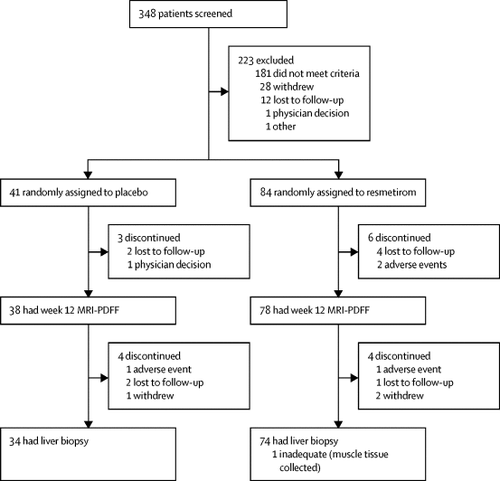
MGL-3196-05 was a 36-week randomised, double-blind, placebo-controlled study at 25 centres in the USA. Adults with biopsy confirmed NASH (fibrosis stages 1-3) and hepatic fat fraction of at least 10% at baseline when assessed by MRI-proton density fat fraction (MRI-PDFF) were eligible. Patients were randomly assigned 2:1 by a computer-based system to receive resmetirom 80 mg or matching placebo, orally once a day. Serial hepatic fat measurements were obtained at weeks 12 and 36, and a second liver biopsy was obtained at week 36. The primary endpoint was relative change in MRI-PDFF assessed hepatic fat compared with placebo at week 12 in patients who had both a baseline and week 12 MRI-PDFF. This trial is registered with ClinicalTrials.gov, number NCT02912260.
FINDINGS
348 patients were screened and 84 were randomly assigned to resmetirom and 41 to placebo at 18 sites in the USA. Resmetirom-treated patients (n=78) showed a relative reduction of hepatic fat compared with placebo (n=38) at week 12 (-32·9% resmetirom vs -10·4% placebo; least squares mean difference -22·5%, 95% CI -32·9 to -12·2; p<0·0001) and week 36 (-37·3% resmetirom [n=74] vs -8·5 placebo [n=34]; -28·8%, -42·0 to -15·7; p<0·0001). Adverse events were mostly mild or moderate and were balanced between groups, except for a higher incidence of transient mild diarrhoea and nausea with resmetirom.
INTERPRETATION
Resmetirom treatment resulted in significant reduction in hepatic fat after 12 weeks and 36 weeks of treatment in patients with NASH. Further studies of resmetirom will allow assessment of safety and effectiveness of resmetirom in a larger number of patients with NASH with the possibility of documenting associations between histological effects and changes in non-invasive markers and imaging.
FUNDING
Madrigal Pharmaceuticals.
中文翻译:

Resmetirom(MGL-3196)用于治疗非酒精性脂肪性肝炎:一项多中心,随机,双盲,安慰剂对照的2期临床试验。
背景技术非酒精性脂肪性肝炎(NASH)的特征在于肝脂肪变性,炎症,肝细胞损伤和进行性肝纤维化。Resmetirom(MGL-3196)是一种肝脏定向的,口服活性的选择性甲状腺激素受体β激动剂,旨在通过增加肝脂肪代谢和降低脂毒性来改善NASH。我们旨在评估瑞司他莫在NASH患者中的安全性和有效性。方法MGL-3196-05是一项在美国25个中心进行的为期36周的随机,双盲,安慰剂对照研究。经MRI质子密度脂肪分数(MRI-PDFF)评估,经活检证实为NASH(纤维化1-3期)且基线时肝脂肪分数至少为10%的成人是合格的。患者通过计算机系统以2:1随机分配,每天口服一次,接受80 mg雷米替林或相匹配的安慰剂。在第12周和第36周进行了系列肝脂肪测量,并在第36周进行了第二次肝活检。主要终点是在基线和基线时均在MRI-PDFF评估的肝脂肪与安慰剂相比在第12周有相对变化。第12周MRI-PDFF。该试验已在ClinicalTrials.gov上注册,编号为NCT02912260。结果在美国的18个地点,对348例患者进行了筛查,其中84例被随机分配给雷米特罗,41例被分配给安慰剂。在第12周时,接受瑞美汀治疗的患者(n = 78)与安慰剂(n = 38)相比,肝脏脂肪相对减少(-32·9%瑞美通与-10·4%安慰剂;最小二乘均数差-22·5 %,95%CI -32·9至-12·2; p <0·0001)和第36周(-37·3%瑞司他林[n = 74]与-8·5安慰剂[n = 34];-28 ·8%,-42·0至-15·7; p <0·0001)。不良事件多为轻度或中度,各组之间的不良事件平衡,除了短暂的轻度腹泻和瑞昔洛明的恶心发生率较高。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞美洛美可评估瑞美美在许多NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞司他罗将允许评估瑞司他罗在大量NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞美洛美可评估瑞美美在许多NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。
更新日期:2019-11-29
中文翻译:

Resmetirom(MGL-3196)用于治疗非酒精性脂肪性肝炎:一项多中心,随机,双盲,安慰剂对照的2期临床试验。
背景技术非酒精性脂肪性肝炎(NASH)的特征在于肝脂肪变性,炎症,肝细胞损伤和进行性肝纤维化。Resmetirom(MGL-3196)是一种肝脏定向的,口服活性的选择性甲状腺激素受体β激动剂,旨在通过增加肝脂肪代谢和降低脂毒性来改善NASH。我们旨在评估瑞司他莫在NASH患者中的安全性和有效性。方法MGL-3196-05是一项在美国25个中心进行的为期36周的随机,双盲,安慰剂对照研究。经MRI质子密度脂肪分数(MRI-PDFF)评估,经活检证实为NASH(纤维化1-3期)且基线时肝脂肪分数至少为10%的成人是合格的。患者通过计算机系统以2:1随机分配,每天口服一次,接受80 mg雷米替林或相匹配的安慰剂。在第12周和第36周进行了系列肝脂肪测量,并在第36周进行了第二次肝活检。主要终点是在基线和基线时均在MRI-PDFF评估的肝脂肪与安慰剂相比在第12周有相对变化。第12周MRI-PDFF。该试验已在ClinicalTrials.gov上注册,编号为NCT02912260。结果在美国的18个地点,对348例患者进行了筛查,其中84例被随机分配给雷米特罗,41例被分配给安慰剂。在第12周时,接受瑞美汀治疗的患者(n = 78)与安慰剂(n = 38)相比,肝脏脂肪相对减少(-32·9%瑞美通与-10·4%安慰剂;最小二乘均数差-22·5 %,95%CI -32·9至-12·2; p <0·0001)和第36周(-37·3%瑞司他林[n = 74]与-8·5安慰剂[n = 34];-28 ·8%,-42·0至-15·7; p <0·0001)。不良事件多为轻度或中度,各组之间的不良事件平衡,除了短暂的轻度腹泻和瑞昔洛明的恶心发生率较高。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞美洛美可评估瑞美美在许多NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞司他罗将允许评估瑞司他罗在大量NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。解释在接受NASH的患者治疗12周和36周后,Resmetirom治疗可显着减少肝脂肪。进一步研究瑞美洛美可评估瑞美美在许多NASH患者中的安全性和有效性,并有可能记录组织学效应与非侵入性标志物和影像学改变之间的关联。资金Madrigal Pharmaceuticals。










































 京公网安备 11010802027423号
京公网安备 11010802027423号