PLOS ONE ( IF 3.7 ) Pub Date : 2019-11-12 , DOI: 10.1371/journal.pone.0225205 Abebaw Kebede 1, 2 , Dereje Beyene 2 , Bazezew Yenew 1 , Getu Diriba 1 , Zemedu Mehamd 1 , Ayinalem Alemu 1 , Misikr Amare 1 , Gobena Ameni 3
|
Introduction
In Ethiopia, >300 GeneXpert instruments have been deployed for tuberculosis (TB) testing using the Xpert MTB/RIF cartridge. Implementing quality indicators is necessary for monitoring and evaluating the quality of Xpert MTB/RIF diagnostic services.
Objective
To assess the use of quality indicators for the Xpert MTB/RIF molecular assay in Ethiopia and to compare the findings with the predefined targets described in the literature.
Methods
Clinical specimens collected from patients with suspected TB were subjected to Xpert MTB/RIF testing at the National TB Reference Laboratory (NTRL) between January and December 2018. Data were collected from GeneXpert software and Laboratory Information System (LIS) databases. Quality indicators were calculated and analyzed. Bivariate and multivariate analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, Illinois, USA).
Results
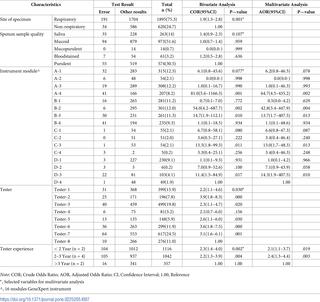
Of the 2515 specimens tested, 2274 (90.4%) had successful test results; 18.2% were positive for Mycobacterium tuberculosis (MTB). Among MTB positives (n = 413), 4.8% and 1.0% were rifampicin (RIF)-resistant and RIF-indeterminate cases, respectively. Unsuccessful results were 241 (9.6%); 8.9% of the total number of tests were errors, 0.04% had invalid results and 0.6% ‘no result’. The most frequent error was probe check failure (error 5007). Instrument module A4, B2, B3, C3, and D3 (p<0.05) and tester experience (p<0.05) had a statistically significant association with errors in multivariate analysis. Additional 42 MTB cases (9.2% of the total cases) were detected among unsuccessful results by follow-up tests. Sixty-four percent of the initial test results were released within the turnaround time (TAT) ≤24 hours.
Conclusion
Most of the quality indicators for the Xpert MTB/RIF molecular assay were maintained within the targets. However, the error rate and TAT were out of the targets. Defective modules and lacking experience were the factors affecting successful test outcomes.
中文翻译:

监测埃塞俄比亚 Xpert MTB/RIF 分子检测的质量指标
介绍
在埃塞俄比亚,已部署超过 300 台 GeneXpert 仪器使用 Xpert MTB/RIF 盒进行结核病 (TB) 检测。实施质量指标对于监控和评估 Xpert MTB/RIF 诊断服务的质量是必要的。
客观的
评估埃塞俄比亚 Xpert MTB/RIF 分子检测质量指标的使用情况,并将研究结果与文献中描述的预定义目标进行比较。
方法
2018 年 1 月至 12 月期间,从疑似结核病患者采集的临床标本在国家结核病参比实验室 (NTRL) 进行了 Xpert MTB/RIF 检测。数据收集自 GeneXpert 软件和实验室信息系统 (LIS) 数据库。并对质量指标进行了计算和分析。使用 SPSS 软件版本 20(SPSS Inc.,芝加哥,伊利诺伊州,美国)进行双变量和多变量分析。
结果
在测试的 2515 个样本中,有 2274 个(90.4%)测试结果成功;18.2% 的结核分枝杆菌(MTB)呈阳性。在 MTB 阳性病例 (n = 413) 中,分别有 4.8% 和 1.0% 为利福平 (RIF) 耐药病例和 RIF 不确定病例。不成功的结果有 241 例(9.6%);测试总数中 8.9% 存在错误,0.04% 结果无效,0.6% “无结果”。最常见的错误是探针检查失败(错误 5007)。仪器模块 A4、B2、B3、C3 和 D3 (p<0.05) 和测试人员经验 (p<0.05) 与多变量分析中的错误具有统计显着相关性。在后续检测的不成功结果中,又发现了 42 例 MTB 病例(占总病例的 9.2%)。64% 的初始测试结果在周转时间 (TAT) ≤ 24 小时内发布。
结论
Xpert MTB/RIF 分子测定的大部分质量指标均保持在目标范围内。然而,错误率和 TAT 都超出了目标。模块缺陷和经验不足是影响测试成功的因素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号