Nature Metabolism ( IF 18.9 ) Pub Date : 2019-11-11 , DOI: 10.1038/s42255-019-0137-5 Chenran Wang 1 , Michael A Haas 1 , Fuchun Yang 1 , Syn Yeo 1 , Takako Okamoto 1 , Song Chen 1 , Jian Wen 1, 2 , Pranjal Sarma 1 , David R Plas 1 , Jun-Lin Guan 1
|
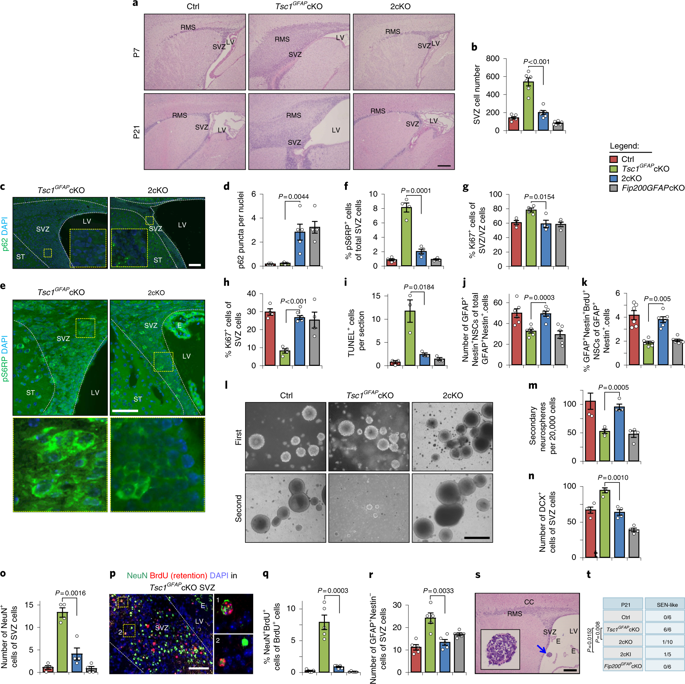
Although mammalian target of rapamycin 1 (mTORC1) negatively regulates autophagy in cultured cells, how autophagy impacts mTORC1 signalling, in particular in an in vivo setting, is less clear. Here we show that autophagy supports mTORC1 hyperactivation in neural stem cells (NSCs) lacking tuberous sclerosis complex subunit 1 (Tsc1), thereby promoting defects in NSC maintenance, differentiation and tumourigenesis, and the formation of the neurodevelopmental lesion of tuberous sclerosis complex (TSC). Analysing mice that lack Tsc1 and the essential autophagy gene Rb1-inducible coiled-coil 1 (Rb1cc1, also called Fip200) in NSCs, we find that TSC-deficient cells require autophagy to maintain mTORC1 hyperactivation under energy-stress conditions, likely to provide free fatty acids via lipophagy to serve as an alternative energy source for OXPHOS. In vivo, inhibition of lipophagy or its downstream catabolic pathway reverses defective phenotypes caused by Tsc1-null NSCs and reduces tumourigenesis in mouse models. These results reveal a cooperative function of selective autophagy in coupling energy availability with TSC pathogenesis and suggest a potential new therapeutic strategy to treat people with TSC.
中文翻译:

自噬脂质代谢维持 TSC 缺陷神经干细胞中的 mTORC1 活性。
尽管哺乳动物雷帕霉素 1 靶标 (mTORC1) 负向调节培养细胞中的自噬,但自噬如何影响 mTORC1 信号传导,特别是在体内环境中,尚不清楚。在这里,我们表明自噬支持 mTORC1 在缺乏结节性硬化症复合体亚基 1 (Tsc1) 的神经干细胞 (NSCs) 中过度活化,从而促进 NSC 维持、分化和肿瘤发生的缺陷,以及结节性硬化症复合体 (TSC) 神经发育病变的形成. 分析缺乏 Tsc1 和必需的自噬基因 Rb1 诱导型卷曲螺旋 1(Rb1cc1,也称为Fip200) 在 NSCs 中,我们发现 TSC 缺陷细胞需要自噬来维持 mTORC1 在能量应激条件下的过度活化,可能通过自噬提供游离脂肪酸作为 OXPHOS 的替代能源。在体内,抑制脂肪吞噬或其下游分解代谢途径可逆转由 Tsc1-null NSC 引起的缺陷表型,并减少小鼠模型中的肿瘤发生。这些结果揭示了选择性自噬在将能量可用性与 TSC 发病机制结合起来的协同作用,并提出了一种治疗 TSC 患者的潜在新治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号