Ageing Research Reviews ( IF 12.5 ) Pub Date : 2019-11-11 , DOI: 10.1016/j.arr.2019.100980 Gregory Livshits 1 , Alexander Kalinkovich 2
|
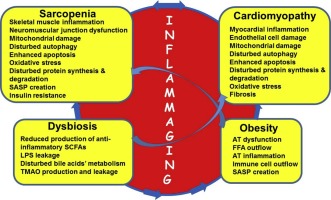
Sarcopenia, obesity and their coexistence, obese sarcopenia (OBSP) as well as atherosclerosis-related cardio-vascular diseases (ACVDs), including chronic heart failure (CHF), are among the greatest public health concerns in the ageing population. A clear age-dependent increased prevalence of sarcopenia and OBSP has been registered in CHF patients, suggesting mechanistic relationships. Development of OBSP could be mediated by a crosstalk between the visceral and subcutaneous adipose tissue (AT) and the skeletal muscle under conditions of low-grade local and systemic inflammation, inflammaging. The present review summarizes the emerging data supporting the idea that inflammaging may serve as a mutual mechanism governing the development of sarcopenia, OBSP and ACVDs. In support of this hypothesis, various immune cells release pro-inflammatory mediators in the skeletal muscle and myocardium. Subsequently, the endothelial structure is disrupted, and cellular processes, such as mitochondrial activity, mitophagy, and autophagy are impaired. Inflamed myocytes lose their contractile properties, which is characteristic of sarcopenia and CHF. Inflammation may increase the risk of ACVD events in a hyperlipidemia-independent manner. Significant reduction of ACVD event rates, without the lowering of plasma lipids, following a specific targeting of key pro-inflammatory cytokines confirms a key role of inflammation in ACVD pathogenesis. Gut dysbiosis, an imbalanced gut microbial community, is known to be deeply involved in the pathogenesis of age-associated sarcopenia and ACVDs by inducing and supporting inflammaging. Dysbiosis induces the production of trimethylamine-N-oxide (TMAO), which is implicated in atherosclerosis, thrombosis, metabolic syndrome, hypertension and poor CHF prognosis. In OBSP, AT dysfunction and inflammation induce, in concert with dysbiosis, lipotoxicity and other pathophysiological processes, thus exacerbating sarcopenia and CHF. Administration of specialized, inflammation pro-resolving mediators has been shown to ameliorate the inflammatory manifestations. Considering all these findings, we hypothesize that sarcopenia, OBSP, CHF and dysbiosis are inflammaging-oriented disorders, whereby inflammaging is common and most probably the causative mechanism driving their pathogenesis.
中文翻译:

发炎是肌肉减少症,肥胖症,心肌病和营养不良的发展和维持的共同基础。
肌肉减少症,肥胖症及其共存,肥胖的肌肉减少症(OBSP)以及动脉粥样硬化相关的心血管疾病(ACVD),包括慢性心力衰竭(CHF),是老龄化人群中最大的公共健康问题。在CHF患者中已发现明显的年龄依赖性肌肉减少症和OBSP患病率升高,提示机制相关。在低度局部和全身性炎症,发炎的情况下,内脏和皮下脂肪组织(AT)与骨骼肌之间的串扰可以介导OBSP的发展。本综述总结了新兴数据,这些数据支持发炎可能是控制肌肉减少症,OBSP和ACVD发生的共同机制。为了支持这一假设,各种免疫细胞在骨骼肌和心肌中释放促炎性介质。随后,内皮结构被破坏,细胞过程如线粒体活性,线粒体吞噬和自噬被破坏。发炎的心肌细胞失去其收缩特性,这是肌肉减少症和CHF的特征。炎症可能以高脂血症无关的方式增加ACVD事件的风险。在特异性靶向关键促炎细胞因子后,ACVD事件发生率显着降低,而血浆脂质却未降低,这证实了炎症在ACVD发病机理中的关键作用。肠道菌群失调是肠道菌群失衡的一种,它通过诱导和支持炎症而深深参与与年龄相关的肌肉减少症和ACVD的发病机理。-N-氧化物(TMAO),与动脉粥样硬化,血栓形成,代谢综合征,高血压和CHF预后不良有关。在OBSP中,AT功能障碍和炎症与营养不良,脂肪毒性和其他病理生理过程共同诱导,从而加剧了肌肉减少症和CHF。已经证明,给予专门的,促炎症的介质可以改善炎症表现。考虑到所有这些发现,我们假设肌肉减少症,OBSP,CHF和营养不良是以发炎为导向的疾病,其中发炎是常见的,最有可能是导致其发病的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号