当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removing scale-forming cations from produced waters
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2019-11-12 , DOI: 10.1039/c9ew00643e Karen Shafer-Peltier 1, 2, 3, 4 , Colton Kenner 1, 3, 4, 5 , Eric Albertson 1, 3, 4, 6 , Ming Chen 1, 1, 2, 3, 4 , Stephen Randtke 1, 3, 4, 5 , Edward Peltier 1, 3, 4, 5
Environmental Science: Water Research & Technology ( IF 3.5 ) Pub Date : 2019-11-12 , DOI: 10.1039/c9ew00643e Karen Shafer-Peltier 1, 2, 3, 4 , Colton Kenner 1, 3, 4, 5 , Eric Albertson 1, 3, 4, 6 , Ming Chen 1, 1, 2, 3, 4 , Stephen Randtke 1, 3, 4, 5 , Edward Peltier 1, 3, 4, 5
Affiliation
|
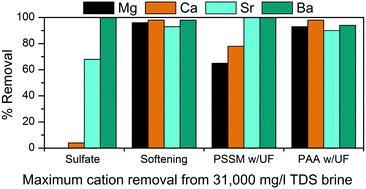
The formation of precipitates (scales) during reinjection limits the reuse of oil and gas production water (produced water) for additional oil recovery. Selective removal strategies that target Ba and Sr, the primary scale-forming cations, would limit produced water treatment costs, reduce waste generation, and increase produced water reuse. A novel treatment technique for targeted Ba and Sr removal, complexation with polyelectrolyte polymers, is compared with chemical precipitation (sulfate addition and precipitative softening) for the removal of Ba and Sr from Kansas oil field brines. Four polymers were examined for cation removal, both with and without ultrafiltration: poly-vinyl sulfonate (PVS), poly(4-styrenesulfonate) (PSS), polyacrylic acid (PAA), and poly(4-styrenesulfonic acid-co-maleic acid) (PSSM). PSSM and PSS were effective for Ba and Sr removal from the lower salinity brine (TDS of 31 000 mg L−1), but exhibited limited Sr removal in the absence of Ba in the high salinity brine (TDS of 92 000 mg l−1). Similar results were achieved in both brines using sulfate addition. PSSM used in conjunction with ultrafiltration removed >99% of initial Sr and Ba from the lower salinity brine, while removing only 65% and 78% of Mg and Ca, respectively. These results compare favorably to precipitative softening, which removed >90% of all divalent cations from the same brine but was less selective for Ba and Sr. PAA plus ultrafiltration removed 58% of Sr (and 68% of Ca) from the high-salinity brine at pH 9. While increased Sr removal can be achieved by polymer-assisted ultrafiltration, further development of this process, including methods for polymer recovery and regeneration, will be needed to improve its performance compared to precipitative softening.
中文翻译:

从采出水中去除水垢形成阳离子
再注入过程中沉淀物(水垢)的形成限制了油气生产水(采出水)的再利用,以用于额外的采油。针对Ba和Sr(形成水垢的主要阳离子)的选择性去除策略将限制采出水的处理成本,减少废物的产生并增加采出水的再利用。将与聚电解质聚合物络合的靶向去除Ba和Sr的新处理技术与从堪萨斯油田盐水中去除Ba和Sr的化学沉淀(添加硫酸盐和沉淀软化)进行了比较。检查用于去除阳离子,具有和不具有超滤四种聚合物:聚磺酸乙烯酯(PVS),聚(4-苯乙烯磺酸盐)(PSS),聚丙烯酸(PAA)和聚(4-苯乙烯酸共-马来酸)(PSSM)。PSSM和PSS可有效去除低盐度盐水(TDS为31 000 mg L -1)中的Ba和Sr ,但在高盐度盐水中Tb为92 000 mg l -1时,在没有Ba的情况下表现出有限的Sr去除。)。使用硫酸盐在两种盐水中都获得了相似的结果。与超滤结合使用的PSSM从低盐度盐水中去除了99%以上的初始Sr和Ba,而分别仅去除了65%和78%的Mg和Ca。这些结果优于沉淀软化,后者从相同的盐水中去除了> 90%的所有二价阳离子,但对Ba和Sr的选择性较低。PAA加超滤去除了高盐度中的58%的Sr(和68%的Ca)。 pH为9的盐水中。虽然可以通过聚合物辅助超滤提高Sr的去除率,但与沉淀软化相比,需要进一步开发此方法,包括聚合物回收和再生的方法,以改善其性能。
更新日期:2019-11-12
中文翻译:

从采出水中去除水垢形成阳离子
再注入过程中沉淀物(水垢)的形成限制了油气生产水(采出水)的再利用,以用于额外的采油。针对Ba和Sr(形成水垢的主要阳离子)的选择性去除策略将限制采出水的处理成本,减少废物的产生并增加采出水的再利用。将与聚电解质聚合物络合的靶向去除Ba和Sr的新处理技术与从堪萨斯油田盐水中去除Ba和Sr的化学沉淀(添加硫酸盐和沉淀软化)进行了比较。检查用于去除阳离子,具有和不具有超滤四种聚合物:聚磺酸乙烯酯(PVS),聚(4-苯乙烯磺酸盐)(PSS),聚丙烯酸(PAA)和聚(4-苯乙烯酸共-马来酸)(PSSM)。PSSM和PSS可有效去除低盐度盐水(TDS为31 000 mg L -1)中的Ba和Sr ,但在高盐度盐水中Tb为92 000 mg l -1时,在没有Ba的情况下表现出有限的Sr去除。)。使用硫酸盐在两种盐水中都获得了相似的结果。与超滤结合使用的PSSM从低盐度盐水中去除了99%以上的初始Sr和Ba,而分别仅去除了65%和78%的Mg和Ca。这些结果优于沉淀软化,后者从相同的盐水中去除了> 90%的所有二价阳离子,但对Ba和Sr的选择性较低。PAA加超滤去除了高盐度中的58%的Sr(和68%的Ca)。 pH为9的盐水中。虽然可以通过聚合物辅助超滤提高Sr的去除率,但与沉淀软化相比,需要进一步开发此方法,包括聚合物回收和再生的方法,以改善其性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号