当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triazole Modified Tetraiodothyroacetic Acid Conjugated to Polyethylene Glycol: High Affinity Thyrointegrin αvβ3 Antagonist with Potent Anticancer Activities in Glioblastoma Multiforme.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.bioconjchem.9b00742 Mehdi Rajabi 1 , Kavitha Godugu 1 , Thangirala Sudha 1 , Dhruba J Bharali 1 , Shaker A Mousa 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.bioconjchem.9b00742 Mehdi Rajabi 1 , Kavitha Godugu 1 , Thangirala Sudha 1 , Dhruba J Bharali 1 , Shaker A Mousa 1
Affiliation
|
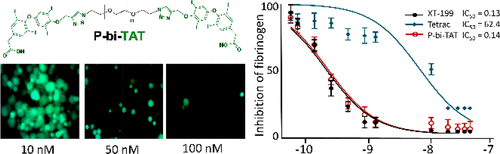
Discovery of bioactive molecules that target integrins has implicated their role in tumor angiogenesis, tumor growth, metastasis, and other pathological angiogenesis processes. Integrins are members of a family of cell surface receptors that play a critical role in the angiogenesis process. Tetraiodothyroacetic acid (tetrac), a deaminated derivative of l-thyroxine (T4), is a "thyrointegrin" antagonist that blocks the actions of l-triiodothyronine (T3) and T4 with an interaction site that is located at or near the RGD recognition site identified on integrin αvβ3's binding pocket (thyrointegrin αvβ3 receptors). We have enhanced the biological activity of a tetrac-based inhibitor via significantly improving its αvβ3 receptor binding affinity by introducing a triazole ring on the outer ring of tetrac and covalently conjugating to polymer to increase the product's hydrophilicity via PEGylation. The product, P-bi-TAT, was restricted from nuclear translocation and demonstrated high blood brain barrier permeability and retention in contrast to the non-PEG conjugated derivative. Results of biological activity indicated that this macromolecule new chemical entity P-bi-TAT has greater than 400-fold potent integrin αvβ3 affinity versus the parent compound tetrac and has potent anticancer/anti-angiogenesis efficacy against glioblastoma multiforme (GBM). P-bi-TAT administered subcutaneously once daily for 21 days at 1-10 mg/kg mouse body weight resulted in a dose-dependent suppression of GBM tumor growth and viability as monitored with IVIS imaging (P < 0.001). GBM tumors had >95% volume loss and maximal loss of GBM cell viability during the 21 days ON-treatment experiment as well as in the 21 days ON followed by 21 days OFF-treatment experiment (P < 0.001). In conclusion, P-bi-TAT is a promising lead clinical candidate effective in the treatment of human GBM.
中文翻译:

与聚乙二醇缀合的三唑修饰四碘甲状腺乙酸:高亲和力甲状腺整合素 αvβ3 拮抗剂,在多形性胶质母细胞瘤中具有强效抗癌活性。
靶向整合素的生物活性分子的发现暗示了它们在肿瘤血管生成、肿瘤生长、转移和其他病理性血管生成过程中的作用。整合素是细胞表面受体家族的成员,在血管生成过程中起着关键作用。四碘甲状腺乙酸 (tetrac) 是 l-甲状腺素 (T4) 的脱氨基衍生物,是一种“甲状腺整合素”拮抗剂,可通过位于或靠近 RGD 识别位点的相互作用位点阻断 l-三碘甲状腺原氨酸 (T3) 和 T4 的作用在整合素 αvβ3 的结合口袋(甲状腺整合素 αvβ3 受体)上鉴定。我们通过在四聚体外环上引入三唑环并与聚合物共价结合以通过聚乙二醇化增加产品的亲水性,显着提高其 αvβ3 受体结合亲和力,从而增强了基于四聚体的抑制剂的生物活性。与非 PEG 共轭衍生物相比,该产品 P-bi-TAT 受到核易位的限制,并表现出高血脑屏障渗透性和保留性。生物活性结果表明,这种大分子新化学实体 P-bi-TAT 对整合素 αvβ3 的亲和力是母体化合物四聚体的 400 多倍,并且对多形性胶质母细胞瘤 (GBM) 具有强大的抗癌/抗血管生成功效。P-bi-TAT 以 1-10 mg/kg 小鼠体重每天一次皮下给药,持续 21 天,导致 GBM 肿瘤生长和活力的剂量依赖性抑制,如 IVIS 成像所监测的 (P < 0.001)。GBM 肿瘤在 21 天的治疗实验以及 21 天的 ON 和 21 天的 OFF-治疗实验期间具有 >95% 的体积损失和 GBM 细胞活力的最大损失(P < 0.001)。总之,P-bi-TAT 是一种有前途的领先临床候选药物,可有效治疗人类 GBM。
更新日期:2019-11-28
中文翻译:

与聚乙二醇缀合的三唑修饰四碘甲状腺乙酸:高亲和力甲状腺整合素 αvβ3 拮抗剂,在多形性胶质母细胞瘤中具有强效抗癌活性。
靶向整合素的生物活性分子的发现暗示了它们在肿瘤血管生成、肿瘤生长、转移和其他病理性血管生成过程中的作用。整合素是细胞表面受体家族的成员,在血管生成过程中起着关键作用。四碘甲状腺乙酸 (tetrac) 是 l-甲状腺素 (T4) 的脱氨基衍生物,是一种“甲状腺整合素”拮抗剂,可通过位于或靠近 RGD 识别位点的相互作用位点阻断 l-三碘甲状腺原氨酸 (T3) 和 T4 的作用在整合素 αvβ3 的结合口袋(甲状腺整合素 αvβ3 受体)上鉴定。我们通过在四聚体外环上引入三唑环并与聚合物共价结合以通过聚乙二醇化增加产品的亲水性,显着提高其 αvβ3 受体结合亲和力,从而增强了基于四聚体的抑制剂的生物活性。与非 PEG 共轭衍生物相比,该产品 P-bi-TAT 受到核易位的限制,并表现出高血脑屏障渗透性和保留性。生物活性结果表明,这种大分子新化学实体 P-bi-TAT 对整合素 αvβ3 的亲和力是母体化合物四聚体的 400 多倍,并且对多形性胶质母细胞瘤 (GBM) 具有强大的抗癌/抗血管生成功效。P-bi-TAT 以 1-10 mg/kg 小鼠体重每天一次皮下给药,持续 21 天,导致 GBM 肿瘤生长和活力的剂量依赖性抑制,如 IVIS 成像所监测的 (P < 0.001)。GBM 肿瘤在 21 天的治疗实验以及 21 天的 ON 和 21 天的 OFF-治疗实验期间具有 >95% 的体积损失和 GBM 细胞活力的最大损失(P < 0.001)。总之,P-bi-TAT 是一种有前途的领先临床候选药物,可有效治疗人类 GBM。









































 京公网安备 11010802027423号
京公网安备 11010802027423号