当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Electrogenerated Cyanomethyl Anion: An Old Base Still Smart.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.accounts.9b00465 Isabella Chiarotto 1 , Leonardo Mattiello 1 , Marta Feroci 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.accounts.9b00465 Isabella Chiarotto 1 , Leonardo Mattiello 1 , Marta Feroci 1
Affiliation
|
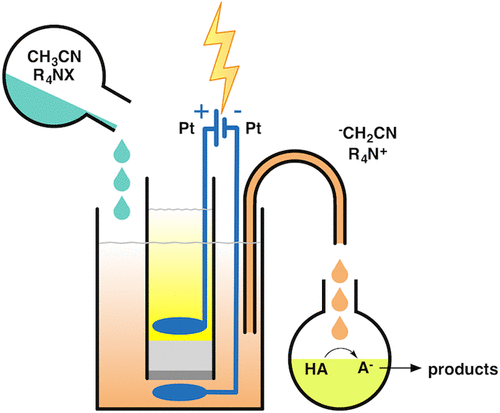
The cathodic reduction of acetonitrile solutions containing a tetraalkylammonium salt leads to the formation of the cyanomethyl anion (-CH2CN, cyanomethanide). This electrolysis can be carried out under very simple experimental conditions (constant-current electrolyses), using various cathodic materials, controlling the amount of base by simply controlling the amount of charge. Despite the fact that the mechanism for this electrochemical reaction is still debated (and it depends on the cathodic material), the outcome of the electrolysis is the formation of a strong base, -CH2CN (pKa 31.3 for acetonitrile in DMSO). The chemical behavior of this electrogenerated base (EGB) strongly depends on its counterion, which in this case is a tetraalkylammonium cation, with a low charge density and thus not coordinated. The very weak interaction between R4N+ and -CH2CN renders the cyanomethyl anion a "naked" ion, and thus highly reactive. In particular, the cyanomethyl anion can react as a base and as a nucleophile. In the first case, it behaves as a strong base and, after deprotonation of a weak acidic substrate, transforms itself into a solvent molecule, acetonitrile, thus generating no byproducts. In the second case, the reactivity as a nucleophile of the cyanomethyl anion obviously depends on the reaction partner. When an electrophile is present in the reaction mixture, a cyanomethylation is obtained (e.g., with aromatic aldehydes, possessing no acidic hydrogen atoms, which undergo nucleophilic attack on the carbonyl carbon atom by -CH2CN); on the contrary, when no reagent is present other than acetonitrile and tetraalkylammonium salt, an attack on the parent molecule leads to the acetonitrile dimer, 3-aminocrotononitrile, which in turn can behave as a base and/or as a nucleophile. In this regard, some authors report that it is preferable to carry out the electrogeneration of the cyanomethyl anion under different experimental conditions, i.e., using an undivided cell and a sacrificial magnesium anode. In this way, a Grignard-type reagent is formed (Mg(CH2CN)2) which highly stabilizes the cyanomethyl anion, preventing its dimerization. It should be noted that in our laboratory the electrogenerated tetraalkylammonium cyanomethanide was extensively used in various reactions (both acid-base and nucleophile-electrophile, vide infra), and in almost no case, the amount of acetonitrile dimer formed exceeded 5%, confirming the validity of this electrochemical methodology to generate a very efficient base. Moreover, when in the reaction mixture both a weak acid and an electrophile are present, the prevalent reactivity of the cyanomethyl anion is as a base, leaving the possibility of a cyanomethylation reaction to those cases in which no acidic substrate is present. We have successfully used the electrogenerated cyanomethyl anion in many base-induced reactions, as the synthesis of the β-lactam ring (chiral or not), the insertion of carbon dioxide into amines and amino alcohols, the activation of elemental sulfur and insertion into carbonyl compounds, the selective alkylation of difunctional compounds, etc.
中文翻译:

电生成的氰甲基阴离子:仍然很老的基地。
含有四烷基铵盐的乙腈溶液的阴极还原可导致氰基甲基阴离子(-CH2CN,氰基甲烷)的形成。该电解可以在非常简单的实验条件下(恒流电解),使用各种阴极材料,通过简单地控制电荷量来控制碱的量来进行。尽管仍在讨论这种电化学反应的机理(取决于阴极材料),但电解的结果是形成了强碱-CH2CN(DMSO中乙腈的pKa 31.3)。这种电生成的碱(EGB)的化学行为在很大程度上取决于其抗衡离子,在这种情况下,它是四烷基铵阳离子,电荷密度低,因此无法配位。R4N +和-CH2CN之间非常弱的相互作用使氰基甲基阴离子成为“裸”离子,因此具有很高的反应性。特别地,氰基甲基阴离子可以作为碱和亲核试剂反应。在第一种情况下,它表现为强碱,在弱酸性底物去质子化后,自身转化为溶剂分子乙腈,因此不产生副产物。在第二种情况下,作为氰基甲基阴离子的亲核试剂的反应性显然取决于反应伙伴。当反应混合物中存在亲电子试剂时,会获得氰基甲基化反应(例如,不具有酸性氢原子的芳香醛,其通过-CH 2 CN对羰基碳原子进行亲核攻击);相反,当除了乙腈和四烷基铵盐以外没有其他试剂存在时,对母体分子的攻击会导致乙腈二聚体3-氨基丁烯腈,而乙腈又可以充当碱基和/或亲核试剂。在这方面,一些作者报道优选在不同的实验条件下,即使用不分格的电池和牺牲性镁阳极进行氰基甲基阴离子的电生成。以此方式,形成格氏试剂(Mg(CH 2 CN)2),其高度稳定氰甲基阴离子,防止其二聚化。应当指出,在我们的实验室中,电生成的氰基四烷基铵氰化物广泛用于各种反应(酸碱和亲核试剂-亲电试剂,见下文),并且在几乎任何情况下,形成的乙腈二聚体的量都不会超过5%,证实了这种电化学方法的有效性,以产生一个非常有效的基础。此外,当在反应混合物中同时存在弱酸和亲电试剂时,氰基甲基阴离子的普遍反应性为碱,从而使那些不存在酸性底物的情况下发生氰基甲基化反应的可能性。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等 氰基甲基阴离子的普遍反应性是作为碱的,对于那些不存在酸性底物的情况,氰基甲基化反应成为可能。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等 氰基甲基阴离子的普遍反应性是作为碱的,对于那些不存在酸性底物的情况,氰基甲基化反应成为可能。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等
更新日期:2019-11-13
中文翻译:

电生成的氰甲基阴离子:仍然很老的基地。
含有四烷基铵盐的乙腈溶液的阴极还原可导致氰基甲基阴离子(-CH2CN,氰基甲烷)的形成。该电解可以在非常简单的实验条件下(恒流电解),使用各种阴极材料,通过简单地控制电荷量来控制碱的量来进行。尽管仍在讨论这种电化学反应的机理(取决于阴极材料),但电解的结果是形成了强碱-CH2CN(DMSO中乙腈的pKa 31.3)。这种电生成的碱(EGB)的化学行为在很大程度上取决于其抗衡离子,在这种情况下,它是四烷基铵阳离子,电荷密度低,因此无法配位。R4N +和-CH2CN之间非常弱的相互作用使氰基甲基阴离子成为“裸”离子,因此具有很高的反应性。特别地,氰基甲基阴离子可以作为碱和亲核试剂反应。在第一种情况下,它表现为强碱,在弱酸性底物去质子化后,自身转化为溶剂分子乙腈,因此不产生副产物。在第二种情况下,作为氰基甲基阴离子的亲核试剂的反应性显然取决于反应伙伴。当反应混合物中存在亲电子试剂时,会获得氰基甲基化反应(例如,不具有酸性氢原子的芳香醛,其通过-CH 2 CN对羰基碳原子进行亲核攻击);相反,当除了乙腈和四烷基铵盐以外没有其他试剂存在时,对母体分子的攻击会导致乙腈二聚体3-氨基丁烯腈,而乙腈又可以充当碱基和/或亲核试剂。在这方面,一些作者报道优选在不同的实验条件下,即使用不分格的电池和牺牲性镁阳极进行氰基甲基阴离子的电生成。以此方式,形成格氏试剂(Mg(CH 2 CN)2),其高度稳定氰甲基阴离子,防止其二聚化。应当指出,在我们的实验室中,电生成的氰基四烷基铵氰化物广泛用于各种反应(酸碱和亲核试剂-亲电试剂,见下文),并且在几乎任何情况下,形成的乙腈二聚体的量都不会超过5%,证实了这种电化学方法的有效性,以产生一个非常有效的基础。此外,当在反应混合物中同时存在弱酸和亲电试剂时,氰基甲基阴离子的普遍反应性为碱,从而使那些不存在酸性底物的情况下发生氰基甲基化反应的可能性。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等 氰基甲基阴离子的普遍反应性是作为碱的,对于那些不存在酸性底物的情况,氰基甲基化反应成为可能。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等 氰基甲基阴离子的普遍反应性是作为碱的,对于那些不存在酸性底物的情况,氰基甲基化反应成为可能。我们已成功地将电生成的氰基甲基阴离子用于许多碱诱导的反应中,例如合成β-内酰胺环(手性或非手性),将二氧化碳插入胺和氨基醇中,活化元素硫并插入羰基中化合物,双官能化合物的选择性烷基化等



























 京公网安备 11010802027423号
京公网安备 11010802027423号