当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Peroxymonocarbonate on the Transformation of Organic Contaminants during Hydrogen Peroxide in Situ Chemical Oxidation
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.estlett.9b00682 Xuejing Yang 1, 2 , Yanghua Duan 1 , Jinling Wang 2, 3 , Hualin Wang 2, 3 , Honglai Liu 3 , David L. Sedlak 1
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.estlett.9b00682 Xuejing Yang 1, 2 , Yanghua Duan 1 , Jinling Wang 2, 3 , Hualin Wang 2, 3 , Honglai Liu 3 , David L. Sedlak 1
Affiliation
|
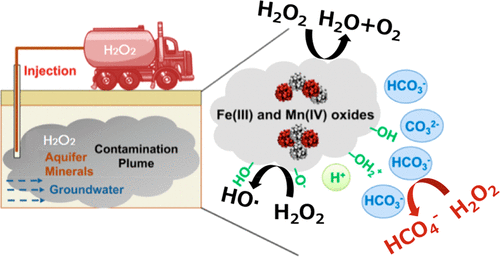
Under the conditions employed when in situ chemical oxidation is used for contaminant remediation, high concentrations of H2O2 (e.g., up to ∼10 M) are typically present. Using 13C NMR, we show that in carbonate-rich systems, these high concentrations of H2O2 result in a reaction with HCO3– to produce peroxymonocarbonate (HCO4–). After formation, HCO4– reacts with phenol to produce di- and trihydroxyl phenols. HCO4– reacts with substituted phenols in a manner consistent with its electrophilic character. Exchanging an electron-donating substituent in the para position of a phenolic compound with an electron-withdrawing group decreased the reaction rate. Results of this study indicate that HCO4– is a potentially important but previously unrecognized oxidative species generated during H2O2in situ chemical oxidation that selectively reacts with electron-rich organic compounds. Under conditions in which HO· formation is inefficient (e.g., relatively high concentration of HCO3–, low total Fe and Mn concentrations), the fraction of the phenolic compounds that is transformed by HCO4– could be similar to or greater than the fraction transformed by HO·. It may be possible to adjust treatment conditions to enhance the formation of HCO4– as a means of accelerating rates of contaminant removal.
中文翻译:

过氧化一氢碳酸盐对过氧化氢原位化学氧化过程中有机污染物转化的影响
在采用原位化学氧化进行污染物修复的条件下,通常会存在高浓度的H 2 O 2(例如,最高约10 M)。使用13 C NMR,我们表明,在富含碳酸盐的系统中,这些高浓度的h的2 ö 2结果与HCO反应3 - ,以产生过一碳酸(HCO 4 - )。形成后,HCO 4 -与苯酚反应生成二-和三羟基酚。HCO 4 –与取代的酚以与其亲电特性一致的方式反应。用具有吸电子基团的酚化合物的对位交换给电子性取代基降低了反应速率。这项研究的结果表明,HCO 4 –是潜在重要但以前无法识别的H 2 O 2原位化学氧化过程中产生的氧化物质,可与富电子有机化合物选择性地发生反应。在HO·形成效率低下的条件下(例如,HCO 3 –的浓度相对较高,Fe和Mn的总浓度较低),HCO转化的酚类化合物分数4 –可能等于或大于HO·转换的分数。它可以是能够调整的处理条件,以提高HCO形成4 -如加速污染物去除速率的手段。
更新日期:2019-11-13
中文翻译:

过氧化一氢碳酸盐对过氧化氢原位化学氧化过程中有机污染物转化的影响
在采用原位化学氧化进行污染物修复的条件下,通常会存在高浓度的H 2 O 2(例如,最高约10 M)。使用13 C NMR,我们表明,在富含碳酸盐的系统中,这些高浓度的h的2 ö 2结果与HCO反应3 - ,以产生过一碳酸(HCO 4 - )。形成后,HCO 4 -与苯酚反应生成二-和三羟基酚。HCO 4 –与取代的酚以与其亲电特性一致的方式反应。用具有吸电子基团的酚化合物的对位交换给电子性取代基降低了反应速率。这项研究的结果表明,HCO 4 –是潜在重要但以前无法识别的H 2 O 2原位化学氧化过程中产生的氧化物质,可与富电子有机化合物选择性地发生反应。在HO·形成效率低下的条件下(例如,HCO 3 –的浓度相对较高,Fe和Mn的总浓度较低),HCO转化的酚类化合物分数4 –可能等于或大于HO·转换的分数。它可以是能够调整的处理条件,以提高HCO形成4 -如加速污染物去除速率的手段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号